User login
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
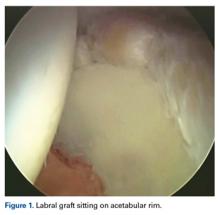
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
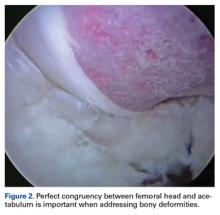
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
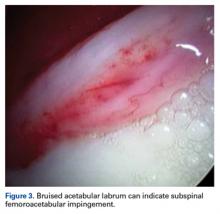
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
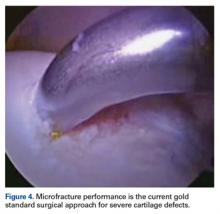
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
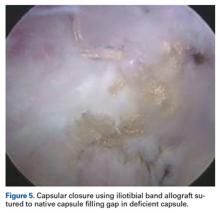
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.