User login
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
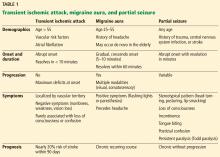
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
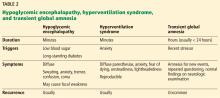
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
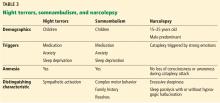
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
KEY POINTS
- Transient ischemic attack, migraine aura, and partial seizures are common and often can be differentiated by their distinctive symptoms.
- Episodes of confusion in a patient with diabetes raise the possibility of hypoglycemic encephalopathy; other possibilities include hyperventilation syndrome and transient global amnesia.
- Daytime sleepiness in a young patient may be due to narcolepsy or parasomnias.