User login
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
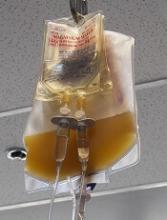
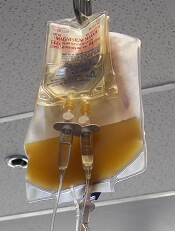
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.