User login
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
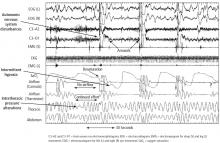
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
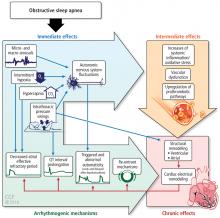
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
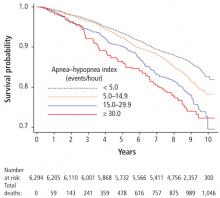
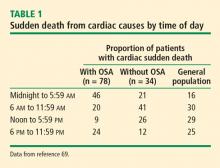
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
KEY POINTS
- Diurnal variations in blood pressure, heart rate, and cardiac events occur during normal sleep.
- While normal sleep may be cardioprotective, sleep apnea disrupts the normal sleep-heart interaction.
- Untreated severe sleep apnea increases the risk for cardiovascular events.
- Treatment with continuous positive airway pressure (CPAP) may reduce the risk of cardiac events based on some data, though randomized studies suggest no improvement in cardiovascular mortality.
- Poor patient adherence to CPAP makes it difficult to evaluate the efficacy of CPAP treatment in clinical trials.