User login
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.
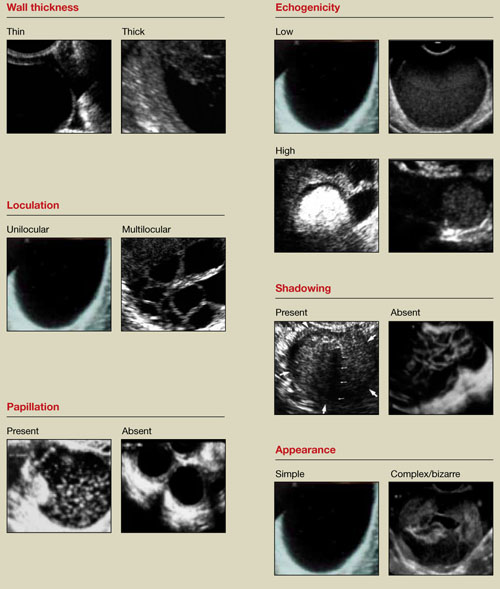
FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6
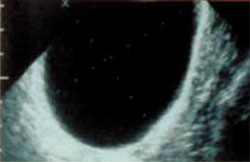
FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).
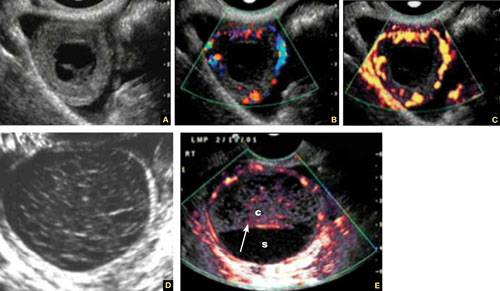
FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.
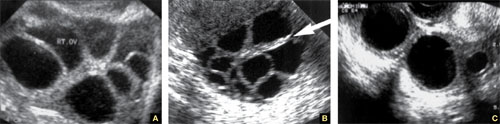
FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.
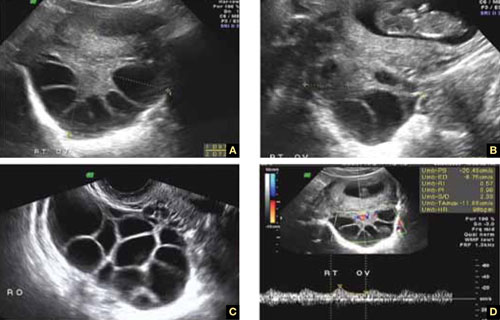
FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7
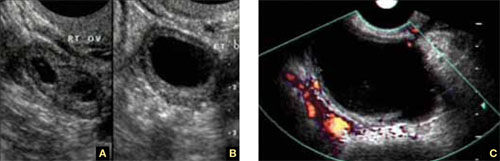
FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.
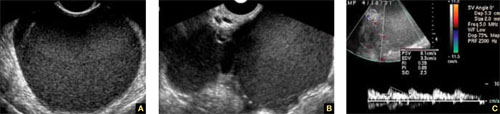
FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.
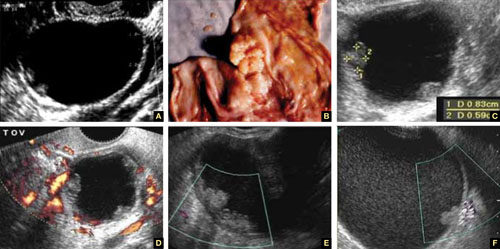
FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.