User login
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).
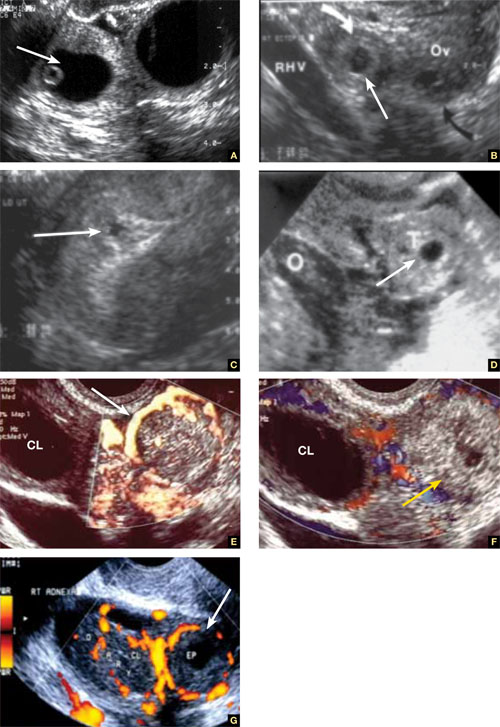
FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).
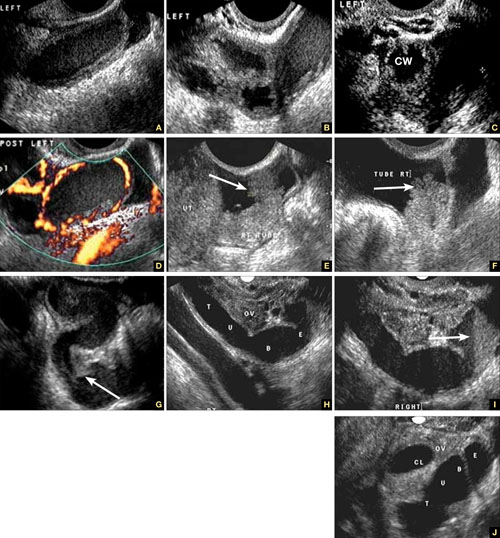
FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.
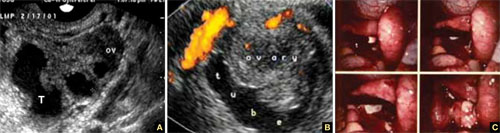
FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).
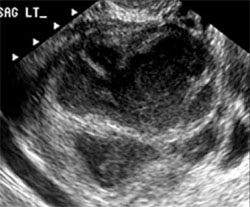
FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).
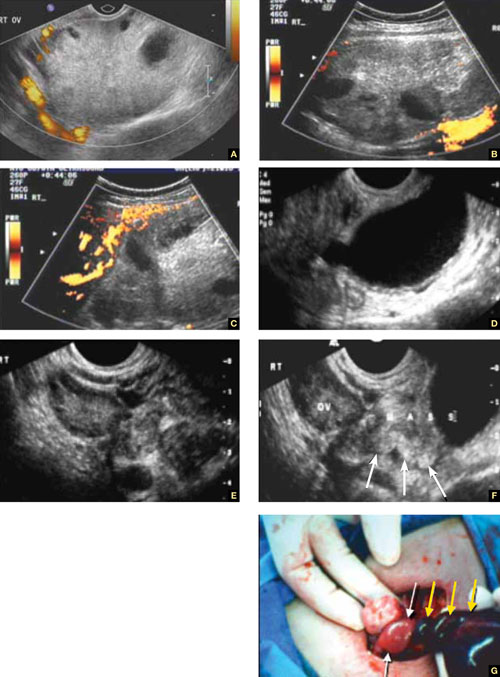
FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.
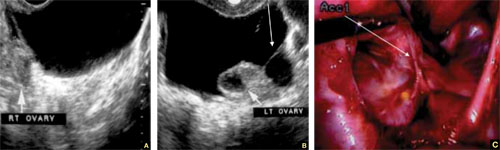
FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.
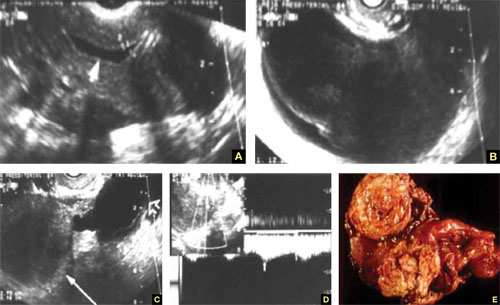
FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.