User login
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
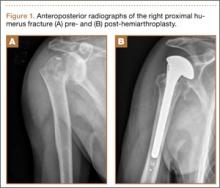
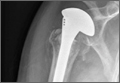
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
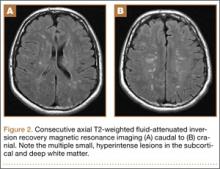
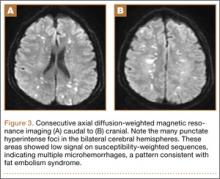
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
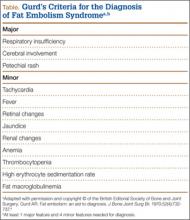
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.