User login
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
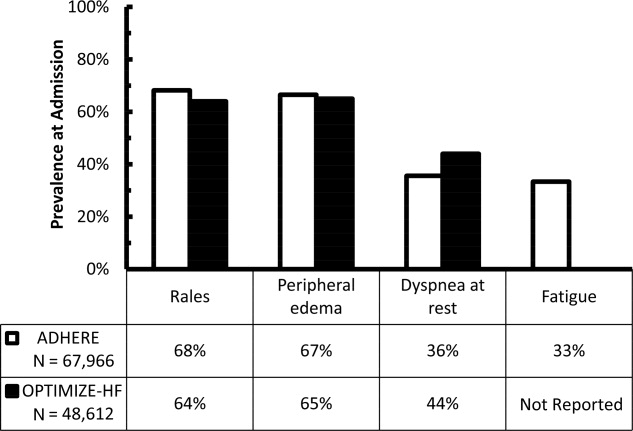
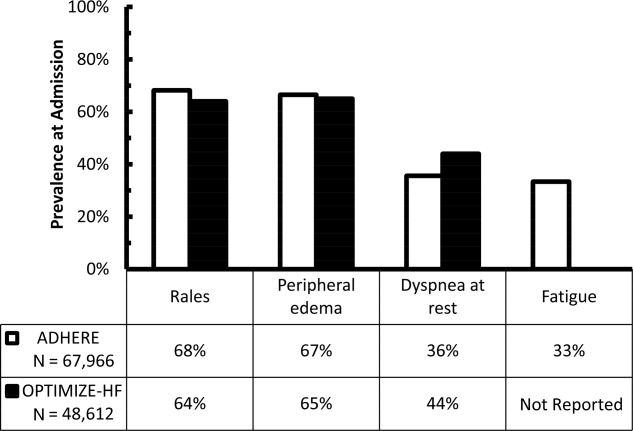
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14
Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
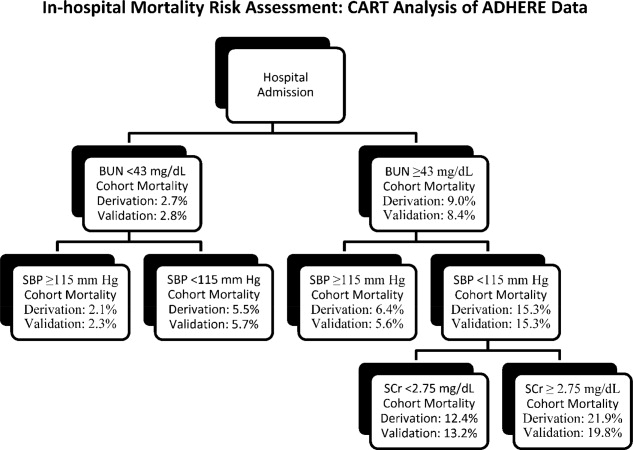
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.
| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14

Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.

| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14

Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.

| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.