User login
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
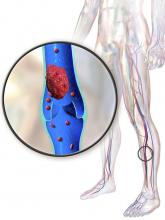
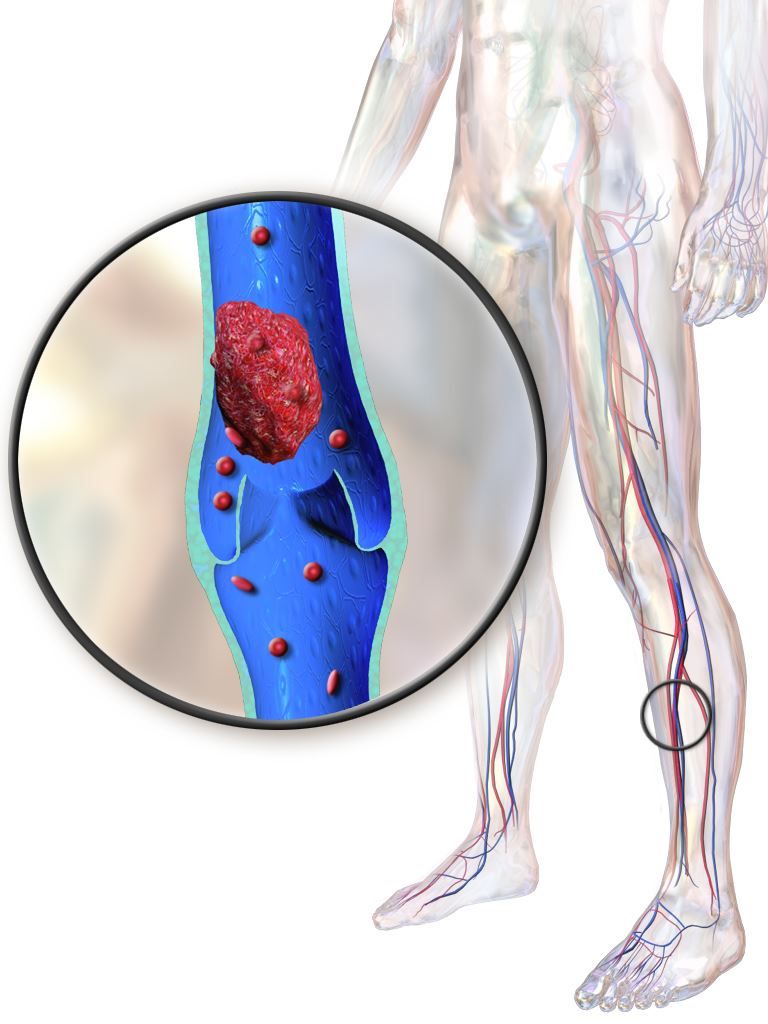
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Rates of post-thrombotic syndrome were 47% in the pharmacomechanical thrombolysis group, and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56).
Data source: A phase 3, multicenter, randomized, open-label, assessor-blinded, controlled clinical trial, including 692 patients with acute proximal deep vein thrombosis.
Disclosures: The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. First author Suresh Vedantham, MD, reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.