User login
IN THIS ARTICLE
• Fasting versus postprandial glucose contribution to A1C
• General glycemic targets for individuals with T2DM
• Sonja's blood glucose log
• Glycemic impact of noninsulin agents available for T2DM
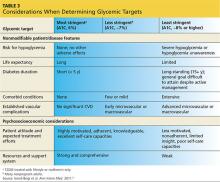
• Considerations when determining glycemic targets
“… Our ability to help others is a source of pride and satisfaction; however, if we listen, really listen to our patients, we may discover that they are also experts, problem-solvers, and teachers. If we allow our patients to also be our teachers, we may someday realize that although we began with knowledge, we ended up with wisdom.” — 1,000 Years of Diabetes Wisdom
(Marrero DG et al, eds)
The pharmacotherapeutic options available for the treatment of type 2 diabetes mellitus (T2DM) have expanded exponentially in the past 15 years. Although this is great news, having so many therapeutic options has led to confusion for both patients and health care providers (HCPs) as they consider which agent or combination of agents is most appropriate for glucose management, while also considering efficacy, safety, adverse effects, patient preferences, and cost.
Current expert recommendations and guidelines provide algorithms that assist the HCP with selecting medications based on safety (avoiding hypoglycemia), adverse-effect profile (eg, weight gain), and efficacy (predicted A1C reduction). These same guidelines also recommend that the choice of antihyperglycemic agent(s) be individualized according to the patient’s health status and personal preferences.
True success in diabetes management requires not only the knowledge and expertise of the clinician, but also the active involvement of the patient as a partner in health care decision making.
Continue for patient presentation/history >>
PATIENT PRESENTATION/HISTORY
We will explore a combined glucose-centric/patient-focused approach with our patient, Sonja.
Sonja is a 38-year-old Latina woman who was diagnosed with T2DM one week ago. She was being closely monitored for diabetes due to a strong family history for T2DM (father, two sisters, and several aunts/uncles affected), high-risk ethnicity, and history of gestational diabetes. Two years ago, when she was told she had prediabetes, she attempted to make appropriate therapeutic lifestyle changes.
Sonja is significantly overweight, with a BMI (29) bordering on obesity. She is inconsistent in her approach to exercise, and her long working hours as a dentist have contributed to a sedentary lifestyle. However, she made a concerted effort to change her diet and successfully lost 18 lb in the past year. Unfortunately, she then experienced considerable stress in her personal life and regained the weight, plus an additional 6 lb.
She presents today to review recent laboratory test results, which include a fasting glucose of 133 mg/dL; serum creatinine (SCr), 1.0 mg/dL; estimated glomerular filtration rate (eGFR), 103 mL/min; A1C, 7.2%; and aspartate transaminase/alanine transaminase (AST/ALT), normal. Sonja says she feels “defeated, frustrated, and helpless” in her attempt to control her weight and thus her inability to avoid T2DM. Fortunately, she wants to change and is determined to do whatever is necessary.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Current guidelines from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) and the American Association of Clinical Endocrinologists (AACE) advise that in addition to a therapeutic lifestyle (adequate physical activity, healthy diet, and weight control), metformin is the drug of choice and is recommended as firstline therapy.1,2
The many available pharmacologic options can make the choice of agents after metformin use an overwhelming task, especially if the HCP has limited experience with them. The 2015 ADA/EASD and AACE algorithms help guide decision making by prioritizing the medications according to efficacy, safety, and adverse-effect profiles.1,2
Emphasis is placed on choosing medications that have low potential for hypoglycemia and, if possible, avoiding medications that may cause weight gain. Additionally, HCPs must take into account patient concerns about adverse effects, convenience/ease of use, mode of administration, and cost. Engaging patients about what is important to them and addressing their beliefs, desires, and fears are key components of individualizing therapy and are essential for successful treatment outcomes.
While Sonja’s current labs suggest that she would be an appropriate candidate for metformin, the drug’s known potential for gastrointestinal (GI) adverse effects is concerning because of Sonja’s underlying history of diarrhea-dominant irritable bowel syndrome (IBS). She remarks that while her IBS is currently controlled, she is wary of developing problems. You respond that extended-release metformin is generally better tolerated than the immediate-release preparations, but it may cost more. She considers this and is willing to try the extended-release option; you instruct her to increase her dose by one 500-mg tablet every week, as tolerated, to reduce the risk for intolerance.
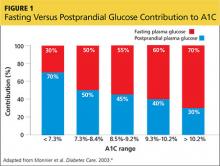
You also discuss blood-glucose testing with her. While she is not taking a medication that will cause hypoglycemia, you explain that structured self-monitoring of blood glucose (SMBG) will provide her immediate feedback about the effects of her lifestyle changes, as well as the effect of the medication, on her blood sugar control.3 Her A1C of 7.2% suggests postprandial glucose (PPG) as a significant contributing factor; thus, it would be beneficial to measure this value regularly (see Figure 14).
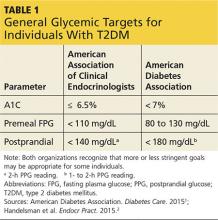
You show Sonja the AACE and ADA therapeutic blood glucose parameters required for optimal glucose control so she can see the impact of her efforts (see Table 11,2). She is willing to test her blood sugar twice daily and agrees to test before and then two hours after a different meal each day (this is known as paired testing).5
Sonja returns two weeks later with her blood glucose log for review (see Figure 2). She is pleased with her improved glucose values but has been unable to exceed 1,000 mg/d due to frequent daytime diarrhea that interferes with work. She requests a change of medication.
Continue for therapeutic considerations >>
THERAPEUTIC CONSIDERATIONS
Glucose-centric
Sonja’s glucose log demonstrates that her blood glucose values are at target with her current dose of extended-release metformin. Based on her glucose patterns and A1C, an agent of choice would be one that best directs its action on postprandial hyperglycemia. Fortunately, at this point in Sonja’s disease state, she should be able to achieve an A1C of < 7% with any of the noninsulin options.
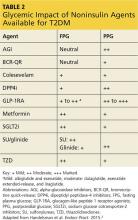
However, when applying the glucose-centric approach, the proper course should be to use an agent that best addresses postprandial hyperglycemia. These agents include glucagon-like peptide 1 receptor agonists (GLP-1RA), dipeptidyl peptidase-4 inhibitors (DPP4i), sulfonylureas (SU), glinide, and α-glucosidase inhibitors (AGI) (see Table 2). Other agents would be less effective in addressing PPG.
Patient-focused
Since Sonja is young, has new-onset T2DM, is otherwise healthy, and has no overt complications from diabetes, her A1C goal should be < 6.5% and perhaps even < 6%, while minimizing the risk for hypoglycemia (see Table 3). However, she continues to be concerned with taking medications associated with any GI-related adverse effects.
The following are discussion points for Sonja regarding the agents approved as monotherapy or as monotherapy when metformin is contraindicated or not tolerated. Although all these classes have potential adverse effects, only GI intolerance and possibility for weight gain are covered here, since these directly pertain to Sonja’s choice of agent.
GLP-1RA (exenatide, liraglutide, exenatide extended-release, albiglutide, dulaglutide).7 This class, along with DPP4i, is also referred to as the incretins. The GLP-1RAs predominately target postprandial hyperglycemia and, to a lesser degree, fasting hyperglycemia—especially when used with the daily options of exenatide and/or liraglutide. The once-weekly options (exenatide extended-release, albiglutide, dulaglutide) have beneficial effects on both fasting and postprandial hyperglycemia.
Though GLP-1RAs are typically well tolerated, the most common associated adverse effects are nausea, which usually resolves in several weeks, and vomiting, which occurs infrequently. The GLP-1RAs are also one of two classes of diabetes medications associated with modest weight loss (the other is sodium glucose cotransporter-2 inhibitors [SGLT2i], to be discussed shortly). An additional benefit of GLP-1RA agents is that they are not associated with hypoglycemia, since they exert their effect in a glucose-dependent manner (ie, only when blood sugar is increased).
While Sonja is not averse to using an injectable agent, she is extremely hesitant to use any agent that may cause GI upset.
DPP4i (sitagliptin, saxagliptin, linagliptin, alogliptin).7 As previously stated, these are in the incretin class along with the GLP-1RAs. They help maintain physiologic levels of endogenous GLP-1, compared with the nearly eightfold pharmacologic level of GLP-1 from the injectable GLP-1RA. DPP4i agents are a physiologically appropriate choice for Sonja, because their effect is primarily on postprandial hyperglycemia. Since these medications also function in a glucose-dependent manner, they are not associated with hypoglycemia.
You explain to Sonja that while the DPP4i agents have a very low GI adverse-effect profile (compared with GLP-1RAs), they are not associated with weight loss but are considered weight neutral.
SU (glyburide, glipizide, glimepiride) and glinides (nateglinide, repaglinide).7 The SU class has a much longer half-life than the glinides and as a result affects both fasting glucose and PPG. The quicker-acting glinides improve PPG extremely well. However, because of the short duration of action, they must be dosed before each meal and sometimes before snacks as well. Since both of these classes stimulate insulin production, they carry a risk for hypoglycemia, but less than for the glinides.8
These agents are generally well tolerated, have a low GI adverse-effect profile, and can be associated with modest weight gain. But the risk for hypoglycemia means they may not be the optimal choice for Sonja.
SGLT2i (canagliflozin, dapagliflozin, empagliflozin).7 The mechanism of action for this class is rather unique in that it reduces re-absorption of glucose by the kidneys, resulting in increased urinary glucose output (glycosuria). This class has been shown to demonstrate modest weight loss. Since increased insulin secretion is not an effect of this class, it carries a very low risk for hypoglycemia.
While SGLT2i medications have a low GI adverse-effect profile, Sonja should be alerted to the associated increased urination, as it may impact her busy work schedule caring for patients.
TZD (rosiglitazone, pioglitazone).7 This is the most effective class for addressing insulin resistance, the key physiologic defect in T2DM. TZD is the only class that has demonstrated long-term A1C reductions (> 5 y).9 The drugs in this class are not associated with hypoglycemia and have a low GI adverse-effect profile. The most common adverse effects are weight gain and fluid retention, which are even more commonly observed in patients also taking insulin. Additionally, there is concern about increased risk for atypical fractures in women, particularly postmenopausal women.
Sonja should be made aware of this potential risk during her postmenopausal years, should she use one of these agents long-term. Currently, however, this would still be a viable option for her since she is early in the course of her disease and likely still has fairly good β-cell function.
AGI (acarbose, miglitol).7 This class is a good choice for directing therapy at postprandial elevations without hypoglycemia and is weight-neutral. Unfortunately, use of these agents has fallen out of favor since they are associated with significant GI adverse effects (ie, bloating, flatulence) and require multiple daily doses, with specific timing before each meal.
Insulin. Insulin is always an option for patients with diabetes, and it is the most effective and natural agent available. However, Sonja’s A1C and glucose pattern—consisting of mild postprandial elevations and near-target fasting glucose—suggest that she does not yet require this medication. Additionally, the risks for hypoglycemia and weight gain make this choice less desirable when other effective therapies are available.
After you have spent time discussing feasible options with Sonja, she decides that she would like to try a DPP4i. You agree and support her decision.
In your discussion, you also reiterate that T2DM is a progressive disease and that Sonja will likely need to use additional agents, possibly even insulin, in the years to come. You encourage her to strive for ongoing good dietary habits, exercise, and weight loss/maintenance, as these measures can lengthen the time before additional diabetes agents are needed.
To assist her with achieving these goals, you refer Sonja to a certified diabetes educator (CDE). The CDE, an integral member of the diabetes management team, will partner with Sonja to develop a plan to successfully implement these necessary lifestyle modifications.
Continue for the conclusion >>
CONCLUSION
Metformin is safe, efficacious, and recommended as a firstline therapy. However, even the best and most effective medication is no good if not taken. Adverse effects, convenience, fears—as perceived by the patient—will ultimately determine treatment success. Therefore, it is often necessary and appropriate to consider other agents in order to meet both the glycemic challenges and the personal choice of patients.
HCPs must incorporate a glucose-centric approach when initiating and advancing noninsulin therapies in order to maximize efficacy, safety, tolerability, and adherence. We must engage patients and involve them as partners in shared decision making. Merging the science of the medications along with realistic preferences of patients solidifies a better provider-patient relationship that will increase the likelihood of meeting glycemic goals and preventing diabetes-related complications and burdens.
REFERENCES
1. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015:38(suppl 1):1-99.
2. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21(suppl 1):1-87.
3. International Diabetes Federation. Guideline: self-monitoring of blood glucose in non–insulin treated type 2 diabetes (2009). www.idf.org/guidelines/self-monitoring. Accessed November 24, 2015.
4. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
5. Parkin CG, Hinnen D, Campbell RK, et al. Effective use of paired testing in type 2 diabetes: practical applications in clinical practice. Diabetes Educ. 2009;35(6):915-927.
6. Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554-559.
7. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed November 20, 2015.
8. Gerich J, Raskin P, Jean-Louis L, et al. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28(9):2093-2099.
9. Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [erratum in N Engl J Med. 2007 Mar 29;356(13):1387-1388]. N Engl J Med. 2006; 355(23):2427-2443.
IN THIS ARTICLE
• Fasting versus postprandial glucose contribution to A1C
• General glycemic targets for individuals with T2DM
• Sonja's blood glucose log
• Glycemic impact of noninsulin agents available for T2DM
• Considerations when determining glycemic targets
“… Our ability to help others is a source of pride and satisfaction; however, if we listen, really listen to our patients, we may discover that they are also experts, problem-solvers, and teachers. If we allow our patients to also be our teachers, we may someday realize that although we began with knowledge, we ended up with wisdom.” — 1,000 Years of Diabetes Wisdom
(Marrero DG et al, eds)
The pharmacotherapeutic options available for the treatment of type 2 diabetes mellitus (T2DM) have expanded exponentially in the past 15 years. Although this is great news, having so many therapeutic options has led to confusion for both patients and health care providers (HCPs) as they consider which agent or combination of agents is most appropriate for glucose management, while also considering efficacy, safety, adverse effects, patient preferences, and cost.
Current expert recommendations and guidelines provide algorithms that assist the HCP with selecting medications based on safety (avoiding hypoglycemia), adverse-effect profile (eg, weight gain), and efficacy (predicted A1C reduction). These same guidelines also recommend that the choice of antihyperglycemic agent(s) be individualized according to the patient’s health status and personal preferences.
True success in diabetes management requires not only the knowledge and expertise of the clinician, but also the active involvement of the patient as a partner in health care decision making.
Continue for patient presentation/history >>
PATIENT PRESENTATION/HISTORY
We will explore a combined glucose-centric/patient-focused approach with our patient, Sonja.
Sonja is a 38-year-old Latina woman who was diagnosed with T2DM one week ago. She was being closely monitored for diabetes due to a strong family history for T2DM (father, two sisters, and several aunts/uncles affected), high-risk ethnicity, and history of gestational diabetes. Two years ago, when she was told she had prediabetes, she attempted to make appropriate therapeutic lifestyle changes.
Sonja is significantly overweight, with a BMI (29) bordering on obesity. She is inconsistent in her approach to exercise, and her long working hours as a dentist have contributed to a sedentary lifestyle. However, she made a concerted effort to change her diet and successfully lost 18 lb in the past year. Unfortunately, she then experienced considerable stress in her personal life and regained the weight, plus an additional 6 lb.
She presents today to review recent laboratory test results, which include a fasting glucose of 133 mg/dL; serum creatinine (SCr), 1.0 mg/dL; estimated glomerular filtration rate (eGFR), 103 mL/min; A1C, 7.2%; and aspartate transaminase/alanine transaminase (AST/ALT), normal. Sonja says she feels “defeated, frustrated, and helpless” in her attempt to control her weight and thus her inability to avoid T2DM. Fortunately, she wants to change and is determined to do whatever is necessary.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Current guidelines from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) and the American Association of Clinical Endocrinologists (AACE) advise that in addition to a therapeutic lifestyle (adequate physical activity, healthy diet, and weight control), metformin is the drug of choice and is recommended as firstline therapy.1,2
The many available pharmacologic options can make the choice of agents after metformin use an overwhelming task, especially if the HCP has limited experience with them. The 2015 ADA/EASD and AACE algorithms help guide decision making by prioritizing the medications according to efficacy, safety, and adverse-effect profiles.1,2
Emphasis is placed on choosing medications that have low potential for hypoglycemia and, if possible, avoiding medications that may cause weight gain. Additionally, HCPs must take into account patient concerns about adverse effects, convenience/ease of use, mode of administration, and cost. Engaging patients about what is important to them and addressing their beliefs, desires, and fears are key components of individualizing therapy and are essential for successful treatment outcomes.
While Sonja’s current labs suggest that she would be an appropriate candidate for metformin, the drug’s known potential for gastrointestinal (GI) adverse effects is concerning because of Sonja’s underlying history of diarrhea-dominant irritable bowel syndrome (IBS). She remarks that while her IBS is currently controlled, she is wary of developing problems. You respond that extended-release metformin is generally better tolerated than the immediate-release preparations, but it may cost more. She considers this and is willing to try the extended-release option; you instruct her to increase her dose by one 500-mg tablet every week, as tolerated, to reduce the risk for intolerance.
You also discuss blood-glucose testing with her. While she is not taking a medication that will cause hypoglycemia, you explain that structured self-monitoring of blood glucose (SMBG) will provide her immediate feedback about the effects of her lifestyle changes, as well as the effect of the medication, on her blood sugar control.3 Her A1C of 7.2% suggests postprandial glucose (PPG) as a significant contributing factor; thus, it would be beneficial to measure this value regularly (see Figure 14).
You show Sonja the AACE and ADA therapeutic blood glucose parameters required for optimal glucose control so she can see the impact of her efforts (see Table 11,2). She is willing to test her blood sugar twice daily and agrees to test before and then two hours after a different meal each day (this is known as paired testing).5
Sonja returns two weeks later with her blood glucose log for review (see Figure 2). She is pleased with her improved glucose values but has been unable to exceed 1,000 mg/d due to frequent daytime diarrhea that interferes with work. She requests a change of medication.
Continue for therapeutic considerations >>
THERAPEUTIC CONSIDERATIONS
Glucose-centric
Sonja’s glucose log demonstrates that her blood glucose values are at target with her current dose of extended-release metformin. Based on her glucose patterns and A1C, an agent of choice would be one that best directs its action on postprandial hyperglycemia. Fortunately, at this point in Sonja’s disease state, she should be able to achieve an A1C of < 7% with any of the noninsulin options.
However, when applying the glucose-centric approach, the proper course should be to use an agent that best addresses postprandial hyperglycemia. These agents include glucagon-like peptide 1 receptor agonists (GLP-1RA), dipeptidyl peptidase-4 inhibitors (DPP4i), sulfonylureas (SU), glinide, and α-glucosidase inhibitors (AGI) (see Table 2). Other agents would be less effective in addressing PPG.
Patient-focused
Since Sonja is young, has new-onset T2DM, is otherwise healthy, and has no overt complications from diabetes, her A1C goal should be < 6.5% and perhaps even < 6%, while minimizing the risk for hypoglycemia (see Table 3). However, she continues to be concerned with taking medications associated with any GI-related adverse effects.
The following are discussion points for Sonja regarding the agents approved as monotherapy or as monotherapy when metformin is contraindicated or not tolerated. Although all these classes have potential adverse effects, only GI intolerance and possibility for weight gain are covered here, since these directly pertain to Sonja’s choice of agent.
GLP-1RA (exenatide, liraglutide, exenatide extended-release, albiglutide, dulaglutide).7 This class, along with DPP4i, is also referred to as the incretins. The GLP-1RAs predominately target postprandial hyperglycemia and, to a lesser degree, fasting hyperglycemia—especially when used with the daily options of exenatide and/or liraglutide. The once-weekly options (exenatide extended-release, albiglutide, dulaglutide) have beneficial effects on both fasting and postprandial hyperglycemia.
Though GLP-1RAs are typically well tolerated, the most common associated adverse effects are nausea, which usually resolves in several weeks, and vomiting, which occurs infrequently. The GLP-1RAs are also one of two classes of diabetes medications associated with modest weight loss (the other is sodium glucose cotransporter-2 inhibitors [SGLT2i], to be discussed shortly). An additional benefit of GLP-1RA agents is that they are not associated with hypoglycemia, since they exert their effect in a glucose-dependent manner (ie, only when blood sugar is increased).
While Sonja is not averse to using an injectable agent, she is extremely hesitant to use any agent that may cause GI upset.
DPP4i (sitagliptin, saxagliptin, linagliptin, alogliptin).7 As previously stated, these are in the incretin class along with the GLP-1RAs. They help maintain physiologic levels of endogenous GLP-1, compared with the nearly eightfold pharmacologic level of GLP-1 from the injectable GLP-1RA. DPP4i agents are a physiologically appropriate choice for Sonja, because their effect is primarily on postprandial hyperglycemia. Since these medications also function in a glucose-dependent manner, they are not associated with hypoglycemia.
You explain to Sonja that while the DPP4i agents have a very low GI adverse-effect profile (compared with GLP-1RAs), they are not associated with weight loss but are considered weight neutral.
SU (glyburide, glipizide, glimepiride) and glinides (nateglinide, repaglinide).7 The SU class has a much longer half-life than the glinides and as a result affects both fasting glucose and PPG. The quicker-acting glinides improve PPG extremely well. However, because of the short duration of action, they must be dosed before each meal and sometimes before snacks as well. Since both of these classes stimulate insulin production, they carry a risk for hypoglycemia, but less than for the glinides.8
These agents are generally well tolerated, have a low GI adverse-effect profile, and can be associated with modest weight gain. But the risk for hypoglycemia means they may not be the optimal choice for Sonja.
SGLT2i (canagliflozin, dapagliflozin, empagliflozin).7 The mechanism of action for this class is rather unique in that it reduces re-absorption of glucose by the kidneys, resulting in increased urinary glucose output (glycosuria). This class has been shown to demonstrate modest weight loss. Since increased insulin secretion is not an effect of this class, it carries a very low risk for hypoglycemia.
While SGLT2i medications have a low GI adverse-effect profile, Sonja should be alerted to the associated increased urination, as it may impact her busy work schedule caring for patients.
TZD (rosiglitazone, pioglitazone).7 This is the most effective class for addressing insulin resistance, the key physiologic defect in T2DM. TZD is the only class that has demonstrated long-term A1C reductions (> 5 y).9 The drugs in this class are not associated with hypoglycemia and have a low GI adverse-effect profile. The most common adverse effects are weight gain and fluid retention, which are even more commonly observed in patients also taking insulin. Additionally, there is concern about increased risk for atypical fractures in women, particularly postmenopausal women.
Sonja should be made aware of this potential risk during her postmenopausal years, should she use one of these agents long-term. Currently, however, this would still be a viable option for her since she is early in the course of her disease and likely still has fairly good β-cell function.
AGI (acarbose, miglitol).7 This class is a good choice for directing therapy at postprandial elevations without hypoglycemia and is weight-neutral. Unfortunately, use of these agents has fallen out of favor since they are associated with significant GI adverse effects (ie, bloating, flatulence) and require multiple daily doses, with specific timing before each meal.
Insulin. Insulin is always an option for patients with diabetes, and it is the most effective and natural agent available. However, Sonja’s A1C and glucose pattern—consisting of mild postprandial elevations and near-target fasting glucose—suggest that she does not yet require this medication. Additionally, the risks for hypoglycemia and weight gain make this choice less desirable when other effective therapies are available.
After you have spent time discussing feasible options with Sonja, she decides that she would like to try a DPP4i. You agree and support her decision.
In your discussion, you also reiterate that T2DM is a progressive disease and that Sonja will likely need to use additional agents, possibly even insulin, in the years to come. You encourage her to strive for ongoing good dietary habits, exercise, and weight loss/maintenance, as these measures can lengthen the time before additional diabetes agents are needed.
To assist her with achieving these goals, you refer Sonja to a certified diabetes educator (CDE). The CDE, an integral member of the diabetes management team, will partner with Sonja to develop a plan to successfully implement these necessary lifestyle modifications.
Continue for the conclusion >>
CONCLUSION
Metformin is safe, efficacious, and recommended as a firstline therapy. However, even the best and most effective medication is no good if not taken. Adverse effects, convenience, fears—as perceived by the patient—will ultimately determine treatment success. Therefore, it is often necessary and appropriate to consider other agents in order to meet both the glycemic challenges and the personal choice of patients.
HCPs must incorporate a glucose-centric approach when initiating and advancing noninsulin therapies in order to maximize efficacy, safety, tolerability, and adherence. We must engage patients and involve them as partners in shared decision making. Merging the science of the medications along with realistic preferences of patients solidifies a better provider-patient relationship that will increase the likelihood of meeting glycemic goals and preventing diabetes-related complications and burdens.
REFERENCES
1. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015:38(suppl 1):1-99.
2. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21(suppl 1):1-87.
3. International Diabetes Federation. Guideline: self-monitoring of blood glucose in non–insulin treated type 2 diabetes (2009). www.idf.org/guidelines/self-monitoring. Accessed November 24, 2015.
4. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
5. Parkin CG, Hinnen D, Campbell RK, et al. Effective use of paired testing in type 2 diabetes: practical applications in clinical practice. Diabetes Educ. 2009;35(6):915-927.
6. Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554-559.
7. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed November 20, 2015.
8. Gerich J, Raskin P, Jean-Louis L, et al. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28(9):2093-2099.
9. Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [erratum in N Engl J Med. 2007 Mar 29;356(13):1387-1388]. N Engl J Med. 2006; 355(23):2427-2443.
IN THIS ARTICLE
• Fasting versus postprandial glucose contribution to A1C
• General glycemic targets for individuals with T2DM
• Sonja's blood glucose log
• Glycemic impact of noninsulin agents available for T2DM
• Considerations when determining glycemic targets
“… Our ability to help others is a source of pride and satisfaction; however, if we listen, really listen to our patients, we may discover that they are also experts, problem-solvers, and teachers. If we allow our patients to also be our teachers, we may someday realize that although we began with knowledge, we ended up with wisdom.” — 1,000 Years of Diabetes Wisdom
(Marrero DG et al, eds)
The pharmacotherapeutic options available for the treatment of type 2 diabetes mellitus (T2DM) have expanded exponentially in the past 15 years. Although this is great news, having so many therapeutic options has led to confusion for both patients and health care providers (HCPs) as they consider which agent or combination of agents is most appropriate for glucose management, while also considering efficacy, safety, adverse effects, patient preferences, and cost.
Current expert recommendations and guidelines provide algorithms that assist the HCP with selecting medications based on safety (avoiding hypoglycemia), adverse-effect profile (eg, weight gain), and efficacy (predicted A1C reduction). These same guidelines also recommend that the choice of antihyperglycemic agent(s) be individualized according to the patient’s health status and personal preferences.
True success in diabetes management requires not only the knowledge and expertise of the clinician, but also the active involvement of the patient as a partner in health care decision making.
Continue for patient presentation/history >>
PATIENT PRESENTATION/HISTORY
We will explore a combined glucose-centric/patient-focused approach with our patient, Sonja.
Sonja is a 38-year-old Latina woman who was diagnosed with T2DM one week ago. She was being closely monitored for diabetes due to a strong family history for T2DM (father, two sisters, and several aunts/uncles affected), high-risk ethnicity, and history of gestational diabetes. Two years ago, when she was told she had prediabetes, she attempted to make appropriate therapeutic lifestyle changes.
Sonja is significantly overweight, with a BMI (29) bordering on obesity. She is inconsistent in her approach to exercise, and her long working hours as a dentist have contributed to a sedentary lifestyle. However, she made a concerted effort to change her diet and successfully lost 18 lb in the past year. Unfortunately, she then experienced considerable stress in her personal life and regained the weight, plus an additional 6 lb.
She presents today to review recent laboratory test results, which include a fasting glucose of 133 mg/dL; serum creatinine (SCr), 1.0 mg/dL; estimated glomerular filtration rate (eGFR), 103 mL/min; A1C, 7.2%; and aspartate transaminase/alanine transaminase (AST/ALT), normal. Sonja says she feels “defeated, frustrated, and helpless” in her attempt to control her weight and thus her inability to avoid T2DM. Fortunately, she wants to change and is determined to do whatever is necessary.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Current guidelines from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) and the American Association of Clinical Endocrinologists (AACE) advise that in addition to a therapeutic lifestyle (adequate physical activity, healthy diet, and weight control), metformin is the drug of choice and is recommended as firstline therapy.1,2
The many available pharmacologic options can make the choice of agents after metformin use an overwhelming task, especially if the HCP has limited experience with them. The 2015 ADA/EASD and AACE algorithms help guide decision making by prioritizing the medications according to efficacy, safety, and adverse-effect profiles.1,2
Emphasis is placed on choosing medications that have low potential for hypoglycemia and, if possible, avoiding medications that may cause weight gain. Additionally, HCPs must take into account patient concerns about adverse effects, convenience/ease of use, mode of administration, and cost. Engaging patients about what is important to them and addressing their beliefs, desires, and fears are key components of individualizing therapy and are essential for successful treatment outcomes.
While Sonja’s current labs suggest that she would be an appropriate candidate for metformin, the drug’s known potential for gastrointestinal (GI) adverse effects is concerning because of Sonja’s underlying history of diarrhea-dominant irritable bowel syndrome (IBS). She remarks that while her IBS is currently controlled, she is wary of developing problems. You respond that extended-release metformin is generally better tolerated than the immediate-release preparations, but it may cost more. She considers this and is willing to try the extended-release option; you instruct her to increase her dose by one 500-mg tablet every week, as tolerated, to reduce the risk for intolerance.
You also discuss blood-glucose testing with her. While she is not taking a medication that will cause hypoglycemia, you explain that structured self-monitoring of blood glucose (SMBG) will provide her immediate feedback about the effects of her lifestyle changes, as well as the effect of the medication, on her blood sugar control.3 Her A1C of 7.2% suggests postprandial glucose (PPG) as a significant contributing factor; thus, it would be beneficial to measure this value regularly (see Figure 14).
You show Sonja the AACE and ADA therapeutic blood glucose parameters required for optimal glucose control so she can see the impact of her efforts (see Table 11,2). She is willing to test her blood sugar twice daily and agrees to test before and then two hours after a different meal each day (this is known as paired testing).5
Sonja returns two weeks later with her blood glucose log for review (see Figure 2). She is pleased with her improved glucose values but has been unable to exceed 1,000 mg/d due to frequent daytime diarrhea that interferes with work. She requests a change of medication.
Continue for therapeutic considerations >>
THERAPEUTIC CONSIDERATIONS
Glucose-centric
Sonja’s glucose log demonstrates that her blood glucose values are at target with her current dose of extended-release metformin. Based on her glucose patterns and A1C, an agent of choice would be one that best directs its action on postprandial hyperglycemia. Fortunately, at this point in Sonja’s disease state, she should be able to achieve an A1C of < 7% with any of the noninsulin options.
However, when applying the glucose-centric approach, the proper course should be to use an agent that best addresses postprandial hyperglycemia. These agents include glucagon-like peptide 1 receptor agonists (GLP-1RA), dipeptidyl peptidase-4 inhibitors (DPP4i), sulfonylureas (SU), glinide, and α-glucosidase inhibitors (AGI) (see Table 2). Other agents would be less effective in addressing PPG.
Patient-focused
Since Sonja is young, has new-onset T2DM, is otherwise healthy, and has no overt complications from diabetes, her A1C goal should be < 6.5% and perhaps even < 6%, while minimizing the risk for hypoglycemia (see Table 3). However, she continues to be concerned with taking medications associated with any GI-related adverse effects.
The following are discussion points for Sonja regarding the agents approved as monotherapy or as monotherapy when metformin is contraindicated or not tolerated. Although all these classes have potential adverse effects, only GI intolerance and possibility for weight gain are covered here, since these directly pertain to Sonja’s choice of agent.
GLP-1RA (exenatide, liraglutide, exenatide extended-release, albiglutide, dulaglutide).7 This class, along with DPP4i, is also referred to as the incretins. The GLP-1RAs predominately target postprandial hyperglycemia and, to a lesser degree, fasting hyperglycemia—especially when used with the daily options of exenatide and/or liraglutide. The once-weekly options (exenatide extended-release, albiglutide, dulaglutide) have beneficial effects on both fasting and postprandial hyperglycemia.
Though GLP-1RAs are typically well tolerated, the most common associated adverse effects are nausea, which usually resolves in several weeks, and vomiting, which occurs infrequently. The GLP-1RAs are also one of two classes of diabetes medications associated with modest weight loss (the other is sodium glucose cotransporter-2 inhibitors [SGLT2i], to be discussed shortly). An additional benefit of GLP-1RA agents is that they are not associated with hypoglycemia, since they exert their effect in a glucose-dependent manner (ie, only when blood sugar is increased).
While Sonja is not averse to using an injectable agent, she is extremely hesitant to use any agent that may cause GI upset.
DPP4i (sitagliptin, saxagliptin, linagliptin, alogliptin).7 As previously stated, these are in the incretin class along with the GLP-1RAs. They help maintain physiologic levels of endogenous GLP-1, compared with the nearly eightfold pharmacologic level of GLP-1 from the injectable GLP-1RA. DPP4i agents are a physiologically appropriate choice for Sonja, because their effect is primarily on postprandial hyperglycemia. Since these medications also function in a glucose-dependent manner, they are not associated with hypoglycemia.
You explain to Sonja that while the DPP4i agents have a very low GI adverse-effect profile (compared with GLP-1RAs), they are not associated with weight loss but are considered weight neutral.
SU (glyburide, glipizide, glimepiride) and glinides (nateglinide, repaglinide).7 The SU class has a much longer half-life than the glinides and as a result affects both fasting glucose and PPG. The quicker-acting glinides improve PPG extremely well. However, because of the short duration of action, they must be dosed before each meal and sometimes before snacks as well. Since both of these classes stimulate insulin production, they carry a risk for hypoglycemia, but less than for the glinides.8
These agents are generally well tolerated, have a low GI adverse-effect profile, and can be associated with modest weight gain. But the risk for hypoglycemia means they may not be the optimal choice for Sonja.
SGLT2i (canagliflozin, dapagliflozin, empagliflozin).7 The mechanism of action for this class is rather unique in that it reduces re-absorption of glucose by the kidneys, resulting in increased urinary glucose output (glycosuria). This class has been shown to demonstrate modest weight loss. Since increased insulin secretion is not an effect of this class, it carries a very low risk for hypoglycemia.
While SGLT2i medications have a low GI adverse-effect profile, Sonja should be alerted to the associated increased urination, as it may impact her busy work schedule caring for patients.
TZD (rosiglitazone, pioglitazone).7 This is the most effective class for addressing insulin resistance, the key physiologic defect in T2DM. TZD is the only class that has demonstrated long-term A1C reductions (> 5 y).9 The drugs in this class are not associated with hypoglycemia and have a low GI adverse-effect profile. The most common adverse effects are weight gain and fluid retention, which are even more commonly observed in patients also taking insulin. Additionally, there is concern about increased risk for atypical fractures in women, particularly postmenopausal women.
Sonja should be made aware of this potential risk during her postmenopausal years, should she use one of these agents long-term. Currently, however, this would still be a viable option for her since she is early in the course of her disease and likely still has fairly good β-cell function.
AGI (acarbose, miglitol).7 This class is a good choice for directing therapy at postprandial elevations without hypoglycemia and is weight-neutral. Unfortunately, use of these agents has fallen out of favor since they are associated with significant GI adverse effects (ie, bloating, flatulence) and require multiple daily doses, with specific timing before each meal.
Insulin. Insulin is always an option for patients with diabetes, and it is the most effective and natural agent available. However, Sonja’s A1C and glucose pattern—consisting of mild postprandial elevations and near-target fasting glucose—suggest that she does not yet require this medication. Additionally, the risks for hypoglycemia and weight gain make this choice less desirable when other effective therapies are available.
After you have spent time discussing feasible options with Sonja, she decides that she would like to try a DPP4i. You agree and support her decision.
In your discussion, you also reiterate that T2DM is a progressive disease and that Sonja will likely need to use additional agents, possibly even insulin, in the years to come. You encourage her to strive for ongoing good dietary habits, exercise, and weight loss/maintenance, as these measures can lengthen the time before additional diabetes agents are needed.
To assist her with achieving these goals, you refer Sonja to a certified diabetes educator (CDE). The CDE, an integral member of the diabetes management team, will partner with Sonja to develop a plan to successfully implement these necessary lifestyle modifications.
Continue for the conclusion >>
CONCLUSION
Metformin is safe, efficacious, and recommended as a firstline therapy. However, even the best and most effective medication is no good if not taken. Adverse effects, convenience, fears—as perceived by the patient—will ultimately determine treatment success. Therefore, it is often necessary and appropriate to consider other agents in order to meet both the glycemic challenges and the personal choice of patients.
HCPs must incorporate a glucose-centric approach when initiating and advancing noninsulin therapies in order to maximize efficacy, safety, tolerability, and adherence. We must engage patients and involve them as partners in shared decision making. Merging the science of the medications along with realistic preferences of patients solidifies a better provider-patient relationship that will increase the likelihood of meeting glycemic goals and preventing diabetes-related complications and burdens.
REFERENCES
1. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015:38(suppl 1):1-99.
2. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21(suppl 1):1-87.
3. International Diabetes Federation. Guideline: self-monitoring of blood glucose in non–insulin treated type 2 diabetes (2009). www.idf.org/guidelines/self-monitoring. Accessed November 24, 2015.
4. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
5. Parkin CG, Hinnen D, Campbell RK, et al. Effective use of paired testing in type 2 diabetes: practical applications in clinical practice. Diabetes Educ. 2009;35(6):915-927.
6. Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554-559.
7. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed November 20, 2015.
8. Gerich J, Raskin P, Jean-Louis L, et al. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28(9):2093-2099.
9. Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [erratum in N Engl J Med. 2007 Mar 29;356(13):1387-1388]. N Engl J Med. 2006; 355(23):2427-2443.