User login
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.
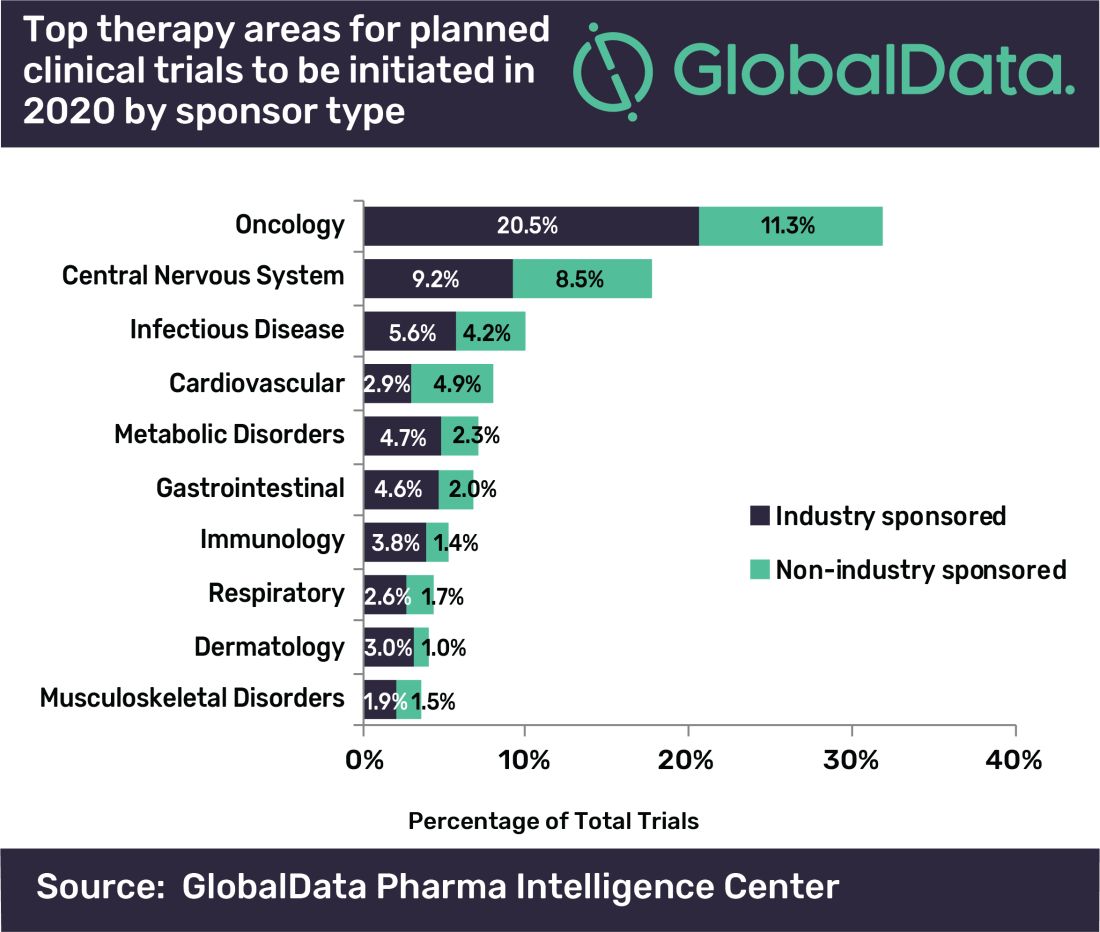
“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.