User login
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
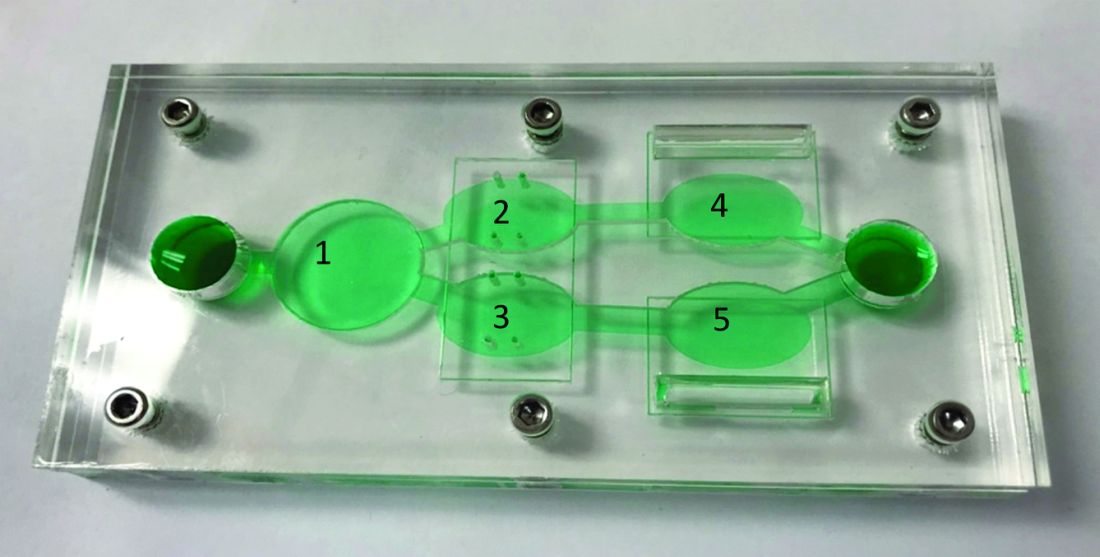
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of various anticancer therapies, investigators report.
“Initially, organ-on-a-chip systems were designed for specific applications with limited ability for reconfiguration and typically with cells from a single organ,” wrote Christopher W. McAleer, PhD, of Hesperos Inc., Orlando, and colleagues. Their report is in Science Translational Medicine.
“To address these issues, a reconfigurable body-on-a-chip system was developed with the capacity to house multiple organ-like tissue constructs,” the authors explained.
The researchers used two different system configurations to evaluate the off-target organ toxicities, metabolism, and efficacy of diclofenac and imatinib (system 1), in addition to tamoxifen (system 2). Both therapies were combined with verapamil in the study.
In system 1, cancer-derived bone marrow cells were cultured with primary hepatocytes, and were analyzed for anti-leukemic activity. In this configuration, both imatinib and diclofenac showed cytostatic activity on cancer progression in the bone marrow cells.
“Liver viability was not affected by imatinib; however, diclofenac reduced liver viability by 30%,” the researchers wrote.
System 2 included a wide variety of cell-lines, including primary hepatocytes, induced pluripotent stem cell-derived cardiomyocytes, a multidrug-resistant vulva cancer line, and a non-multidrug-resistant breast cancer line.
In this configuration, tamoxifen monotherapy and tamoxifen coadministered with verapamil resulted in off-target cardiac toxicities, but did not alter cell viability.
“These systems demonstrate the utility of a human cell–based in vitro culture system to evaluate both on-target efficacy and off-target toxicity for parent drugs and their metabolites,” Dr. McAleer and colleagues wrote.
The researchers acknowledged that the dosing parameters used in the model were acute. As a result, chronic, low-dose treatment strategies may reflect clinical conditions more accurately.
“These systems can augment and reduce the use of animals and increase the efficiency of drug evaluations in preclinical studies,” they concluded.
The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
SOURCE: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A novel multiorgan body-on-a-chip system shows promise to improve the preclinical evaluation of anticancer therapies.
Major finding: Overall, results support the utility of the system to assess both off-target toxicity and on-target efficacy for various anticancer drugs.
Study details: A study exploring the utility of a multi-organ-on-a-chip system to assess safety and effectiveness of anticancer therapies in the preclinical setting.
Disclosures: The study was supported by Hesperos Internal Development funds, the NIH, and Roche. The authors reported financial affiliations with Hesperos and Roche.
Source: McAleer CW et al. Sci Transl Med. 2019 Jun 19. doi: 10.1126/scitranslmed.aav1386.