User login
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
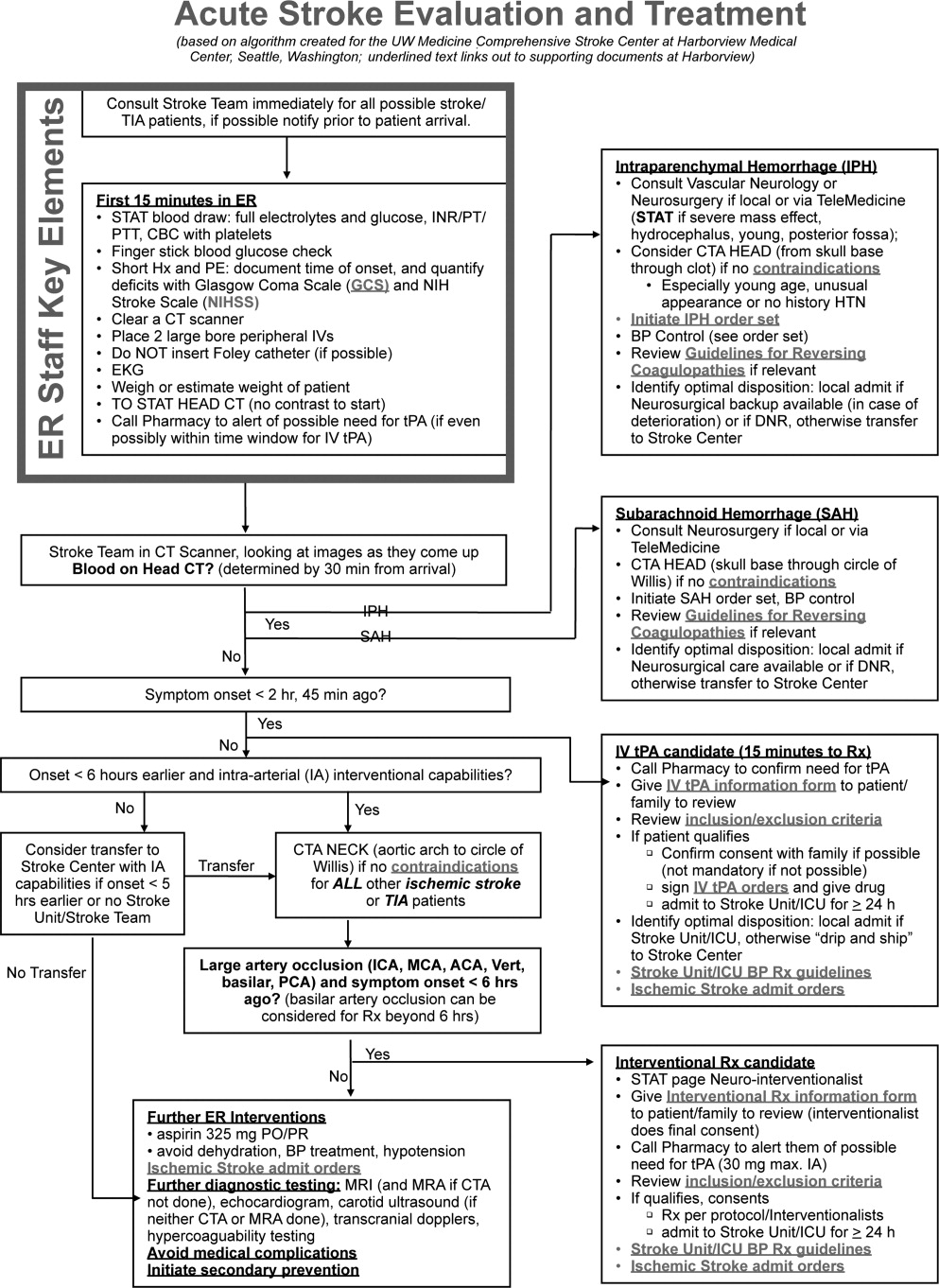
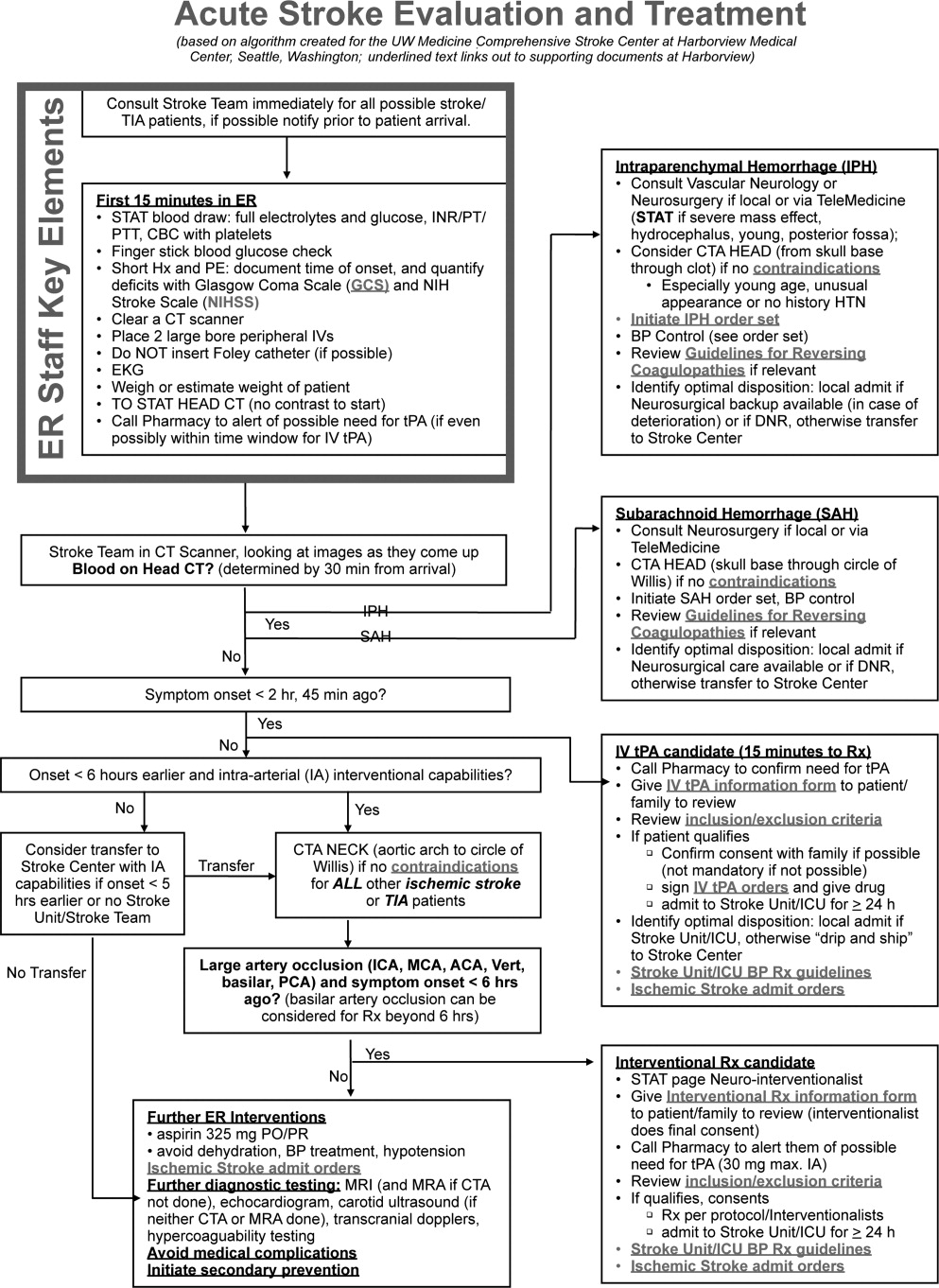
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at
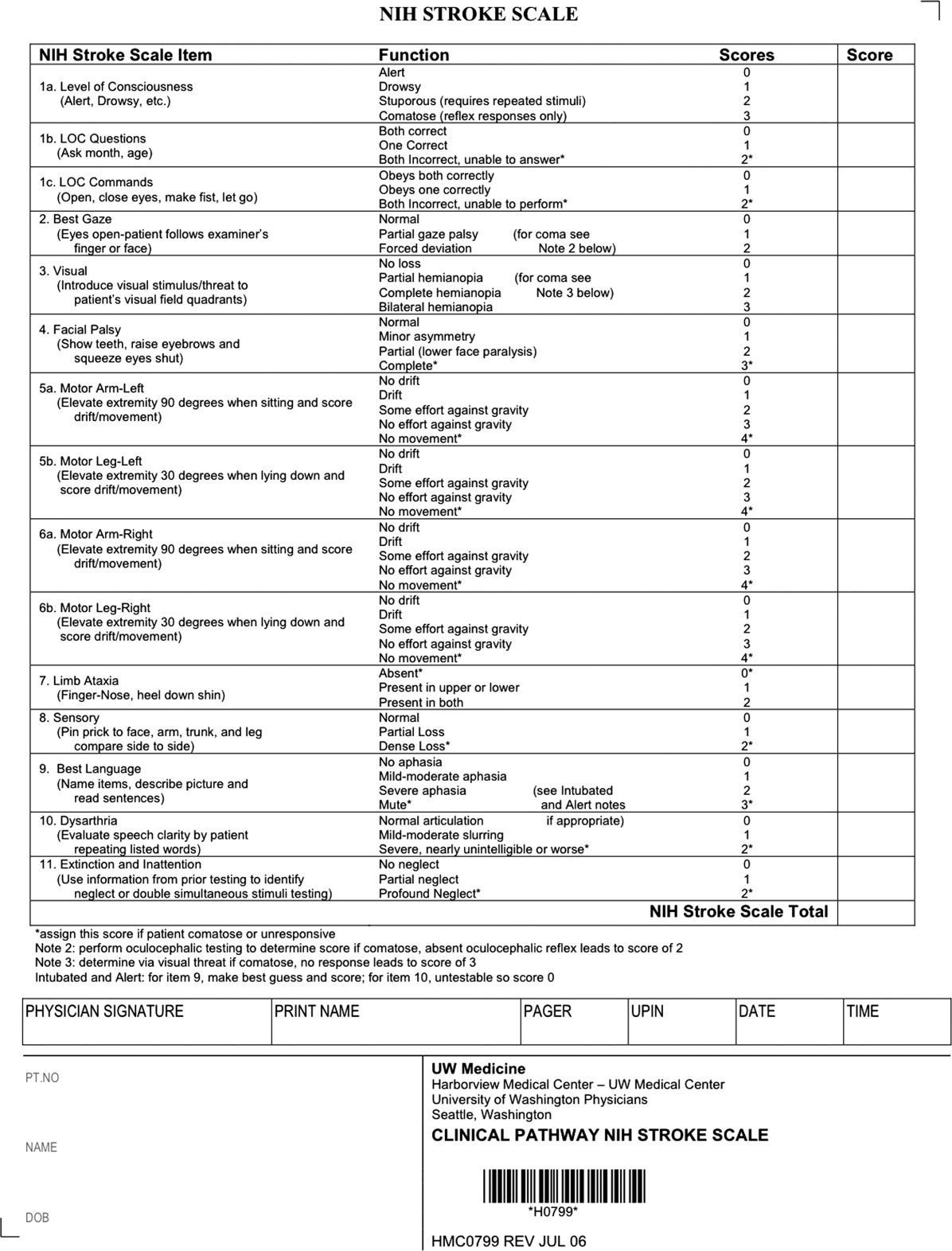
Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
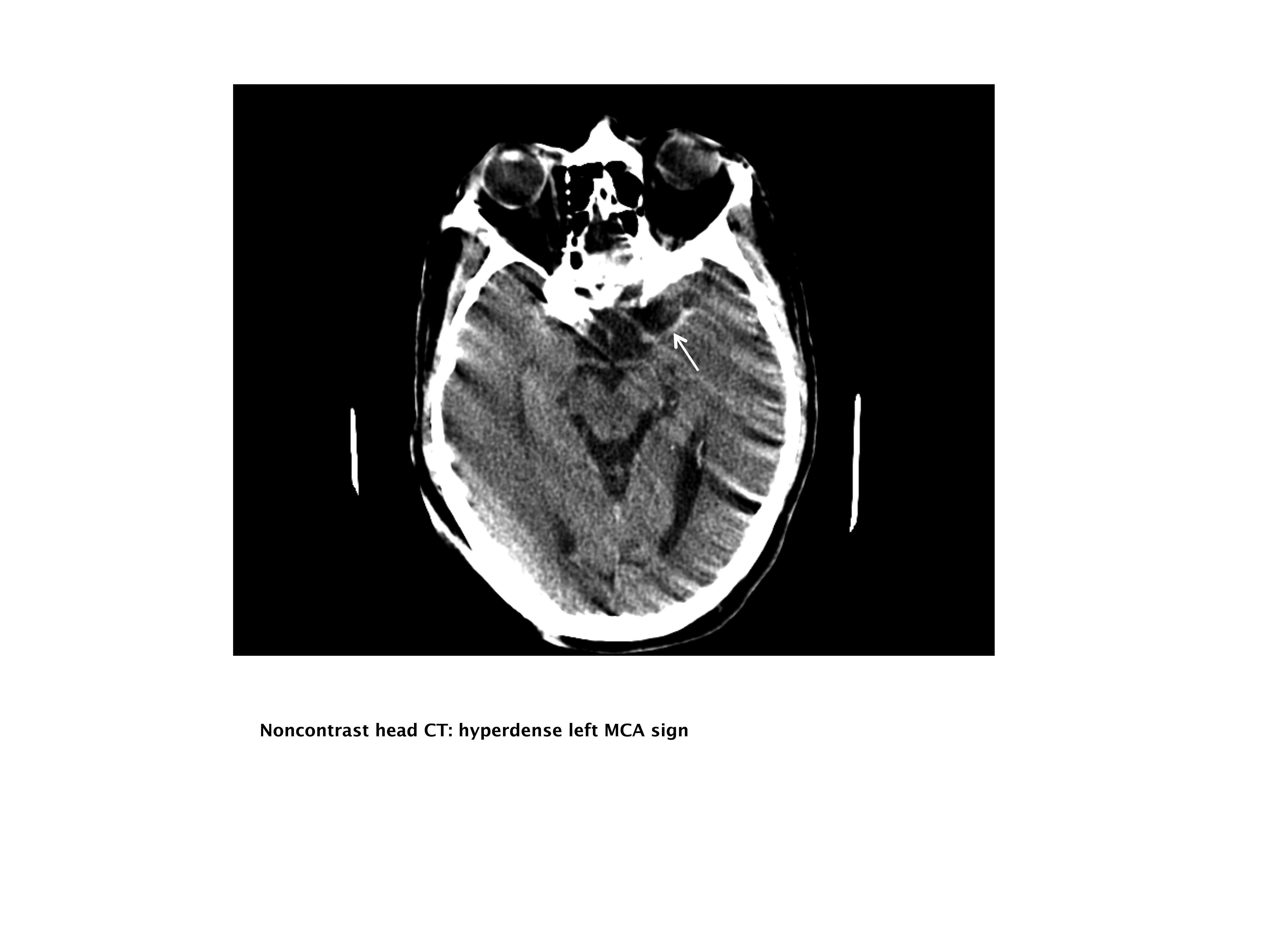
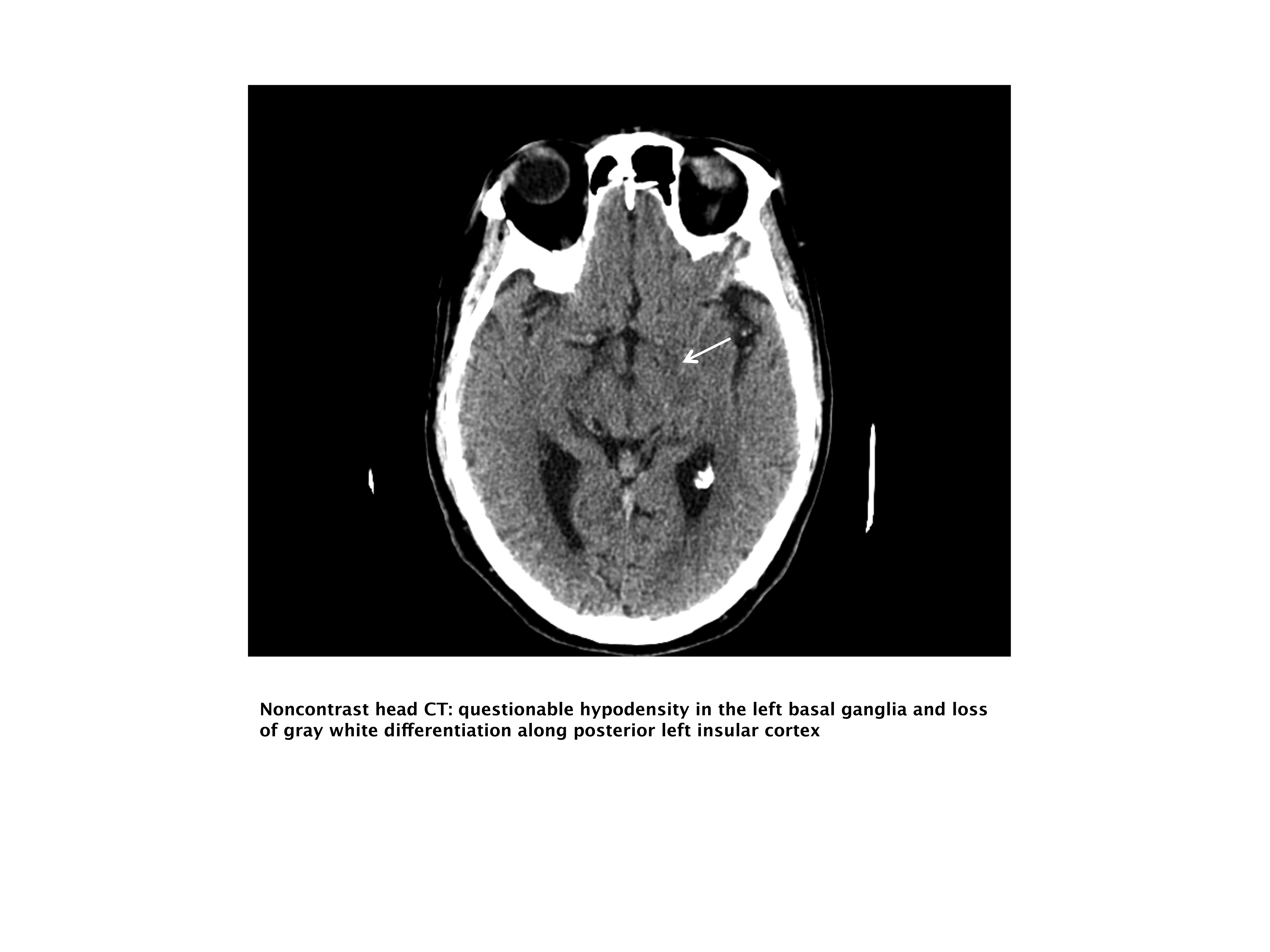
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at


Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at


Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.