User login
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
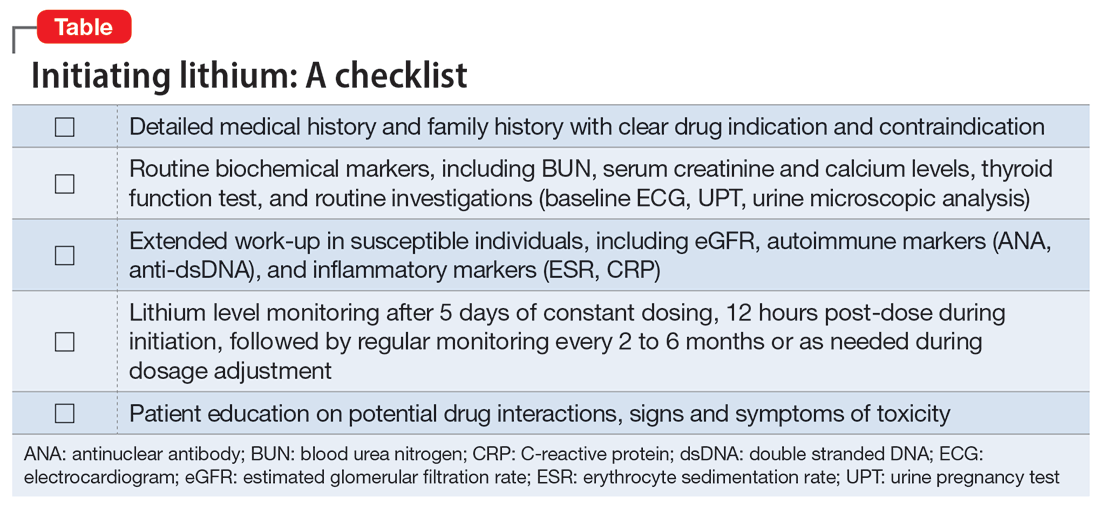
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).
Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
