User login
Introduction
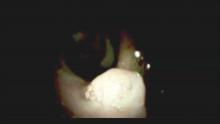
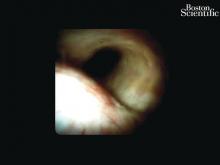
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
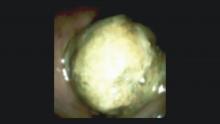
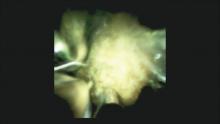
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.