User login
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
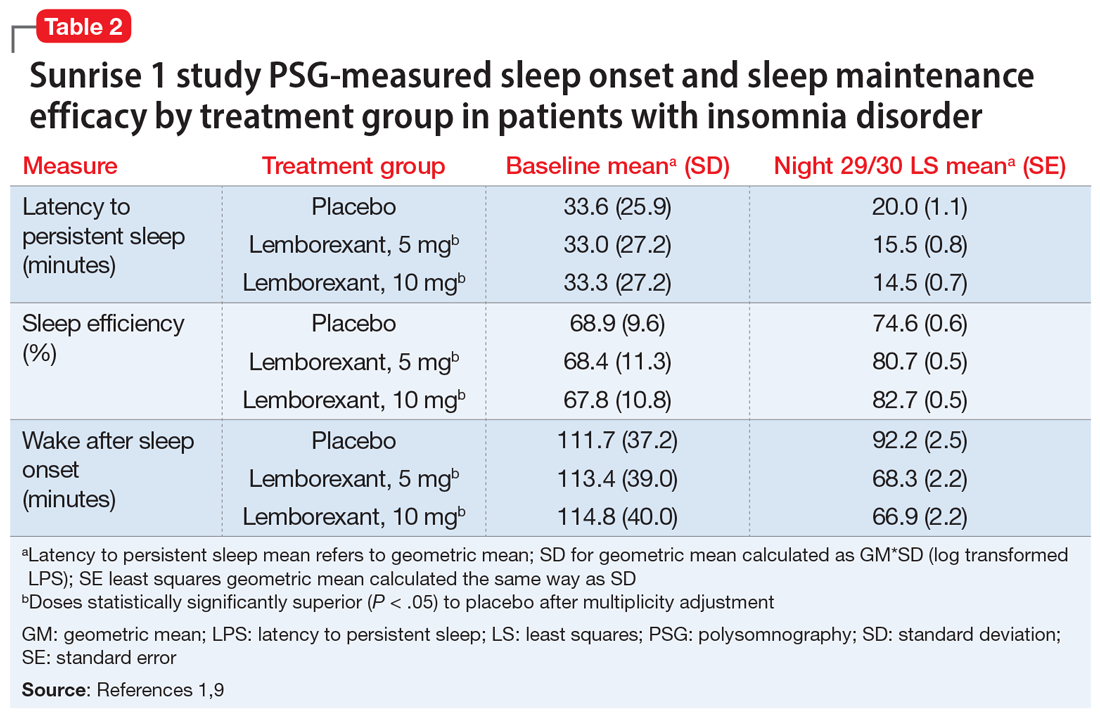
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
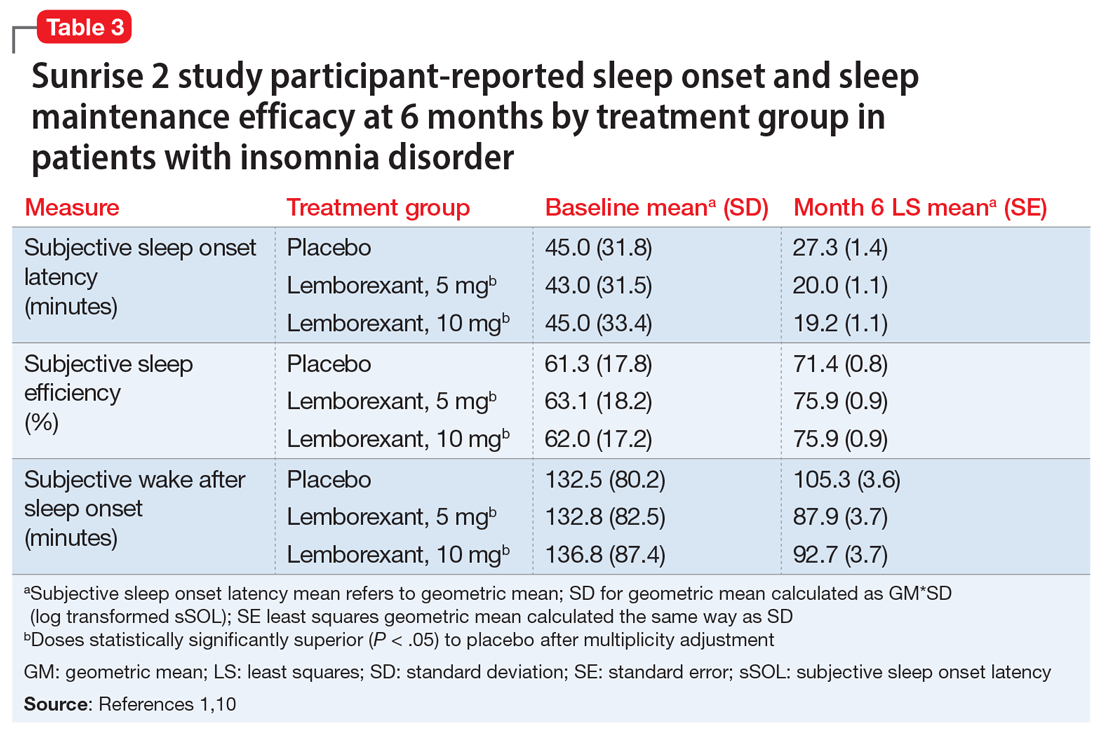
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
Lemborexant, FDA-approved for the treatment of insomnia, has demonstrated efficacy in improving both sleep onset and sleep maintenance.1 This novel compound is now the second approved insomnia medication classed as a dual orexin receptor antagonist (Table 1). This targeted mechanism of action aims to enhance sleep while limiting the adverse effects associated with traditional hypnotics.
Clinical implications
Insomnia symptoms affect approximately one-third of the general population at least occasionally. Approximately 10% of individuals meet DSM-5 criteria for insomnia disorder, which require nighttime sleep difficulty and daytime consequences persisting for a minimum of 3 months.2 The prevalence is considerably higher in patients with chronic medical disorders and comorbid psychiatric conditions, especially mood, anxiety, substance use, and stress- and trauma-related disorders. Clinical guidelines for treating insomnia disorder typically recommend cognitive-behavioral therapy for insomnia as a first choice and FDA-approved insomnia medications as secondary options.3
Currently approved insomnia medications fall into 4 distinct pharmacodynamics categories.4 Benzodiazepine receptor agonist hypnotics include 5 medications with classic benzodiazepine structures (estazolam, flurazepam, quazepam, temazepam, and triazolam) and 3 compounds (eszopiclone, zaleplon, and zolpidem) with alternate structures but similar mechanisms of action. There is 1 melatonin receptor agonist (ramelteon) and 1 histamine receptor antagonist (low-dose doxepin). Joining suvorexant (approved in 2014), lemborexant is the second dual orexin receptor antagonist.
The orexin (also called hypocretin) system was first described in 1998 and its fundamental role in promoting and coordinating wakefulness was quickly established.5 A relatively small number of hypothalamic neurons located in the lateral and perifornical regions produce 2 similar orexin neuropeptides (orexin A and orexin B) with widespread distributions, notably reinforcing the wake-promoting activity of histamine, acetylcholine, dopamine, serotonin, and norepinephrine. Consistent with the typical sleep-wake cycle, orexin release is limited during the nighttime. The orexin neuropeptides interact with 2 G-protein-coupled orexin receptors (OX1R, OX2R).
Animal studies showed that impairment in orexin system activity was associated with symptoms characteristic of narcolepsy, including cataplexy and excessive sleep episodes. Soon after, it was found that humans diagnosed with narcolepsy with cataplexy had markedly low CSF orexin levels.6 This recognition that excessively sleepy people with narcolepsy had a profound decrease in orexin production led to the hypothesis that pharmacologically decreasing orexin activity might be sleep-enhancing for insomnia patients, who presumably are excessively aroused. Numerous compounds soon were evaluated for their potential as orexin receptor antagonists. The efficacy of treating insomnia with a dual orexin receptor antagonist in humans was first reported in 2007 with almorexant, a compound that remains investigational.7 Research continues to investigate both single and dual orexin antagonist molecules for insomnia and other potential indications.
How it works
Unlike most hypnotics, which have widespread CNS depressant effects, lemborexant has a more targeted action in promoting sleep by suppressing the wake drive supported by the orexin system.8 Lemborexant is highly selective for the OX1R and OX2R orexin receptors, where it functions as a competitive antagonist. It is hypothesized that by modulating orexin activity with a receptor antagonist, excessive arousal associated with insomnia can be reduced, thus improving nighttime sleep. The pharmacokinetic properties allow benefits for both sleep onset and maintenance.
Pharmacokinetics
Lemborexant is available in immediate-release tablets with a peak concentration time (Tmax) of approximately 1 to 3 hours after ingestion. When taken after a high-fat and high-calorie meal, there is a delay in the Tmax, a decrease in the maximum plasma concentration (Cmax), and an increase in the concentration area under the curve (AUC0-inf).1
Continue to: Metabolism is primarily through...
Metabolism is primarily through the cytochrome P450 (CYP) 3A4 pathway, and to a lesser extent through CYP3A5. Concomitant use with moderate or strong CYP3A inhibitors or inducers should be avoided, while use with weak CYP3A inhibitors should be limited to the 5-mg dose of lemborexant.
Lemborexant has the potential to induce the metabolism of CYP2B6 substrates, such as bupropion and methadone, possibly leading to reduced efficacy for these medications. Accordingly, the clinical responses to any CYP2B6 substrates should be monitored and dosage adjustments considered.
Concomitant use of lemborexant with alcohol should be avoided because there may be increased impairment in postural stability and memory, in part due to increases in the medication’s Cmax and AUC, as well as the direct effects of alcohol.
Efficacy
In randomized, placebo-controlled trials, lemborexant demonstrated both objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance in patients diagnosed with insomnia disorder.1 The 2 pivotal efficacy studies were:
- Sunrise 1, a 4-week trial with older adults that included laboratory polysomnography (PSG) studies (objective) and patient-reported sleep measures (subjective) on selected nights9
- Sunrise 2, a 6-month trial assessing patient-reported sleep characteristics in adults and older adults.10
Sunrise 1 was performed with older adults with insomnia who were randomized to groups with nightly use of lemborexant, 5 mg (n = 266), lemborexant, 10 mg (n = 269), zolpidem extended-release, 6.25 mg, as an active comparator (n = 263), or placebo (n = 208).9 The age range was 55 to 88 years with a median age of 63 years. Most patients (86.4%) were women. Because this study focused on the assessment of efficacy for treating sleep maintenance difficulty, the inclusion criteria required a subjective report of experiencing a wake time after sleep onset (sWASO) of at least 60 minutes for 3 or more nights per week over the previous 4 weeks. The zolpidem extended-release 6.25 mg comparison was chosen because it has an indication for sleep maintenance insomnia with this recommended dose for older adults.
Continue to: Laboratory PSG monitoring...
Laboratory PSG monitoring was performed for 2 consecutive nights at baseline (before treatment), the first 2 treatment nights, and the final 2 treatment nights (Nights 29 and 30). The primary study endpoint was the change in latency to persistent sleep (LPS) from baseline to the final 2 nights for the lemborexant doses compared with placebo. Additional PSG-based endpoints were similar comparisons for sleep efficiency (percent time asleep during the 8-hour laboratory recording period) and objective wake after sleep onset (WASO) compared with placebo, and WASO during the second half of the night (WASO2H) compared with zolpidem. Patients completed Insomnia Severity Index (ISI) questionnaires at baseline and the end of the treatment to compare disease severity. Subjective assessments were done daily with electronic diary entries that included sleep onset latency (sSOL), sWASO, and subjective sleep efficiency.
In comparison with placebo, both lemborexant doses were associated with significantly improved PSG measures of LPS, WASO, and sleep efficiency during nights 1 and 2 that were maintained through Nights 29 and 30 (Table 21,9). The lemborexant doses also demonstrated significant improvements in WASO2H compared with zolpidem and placebo on the first 2 and final 2 treatment nights. Analyses of the subjective assessments (sSOL, sWASO, and sleep efficiency) compared the baseline with means for the first and the last treatment weeks. At both lemborexant doses, the sSOL was significantly reduced during the first and last weeks compared with placebo and zolpidem. Subjective sleep efficiency was significantly improved at both time points for the lemborexant doses, though these were not significantly different from the zolpidem values. The sWASO values were significantly decreased for both lemborexant doses at both time points compared with placebo. During the first treatment week, both lemborexant doses did not differ significantly from zolpidem in the sWASO change from baseline; however, at the final treatment week, the zolpidem value was significantly improved compared with lemborexant, 5 mg, but not significantly different from lemborexant, 10 mg. The ISI change from baseline to the end of the treatment period showed significant improvement for the lemborexant doses and zolpidem extended-release compared with placebo.
In the Sunrise 2 study, patients who met the criteria for insomnia disorder (age range 18 to 88, mean 55; 68% female) were randomized to groups taking nightly doses of lemborexant, 5 mg (n = 323), lemborexant, 10 mg (n = 323), or placebo (n = 325) for 6 months.10 Inclusion criteria required an sSOL of at least 30 minutes and/or a sWASO of at least 60 minutes 3 times a week or more during the previous 4 weeks. Efficacy was assessed with daily electronic diary entries, with analyses of change from baseline for sSOL (primary endpoint, baseline to end of 6-month study period), sWASO, and patient-reported sleep efficiency (sSEF). Subjective total sleep time (sTST) represented the estimated time asleep during the time in bed. Additional diary assessments related to sleep quality and morning alertness. All of these subjective assessments were compared as 7-day means for the first week of treatment and the last week of each treatment month.
The superiority of lemborexant, 5 mg and 10 mg, compared with placebo was demonstrated by significant improvements in sSOL, sSEF, sWASO, and sTST during the initial week of the treatment period that remained significant at the end of the 6-month placebo-controlled period (Table 31,10). At the end of 6 months, there were significantly more sleep-onset responders and sleep-maintenance responders among patients taking lemborexant compared with those taking placebo. Sleep-onset responders were patients with a baseline sSOL >30 minutes and a mean sSOL ≤20 minutes at the end of the study. Sleep-maintenance responders were participants with a baseline sWASO >60 minutes who at the end of the study had a mean sWASO ≤60 minutes that included a reduction of at least 10 minutes.
Following the 6-month placebo-controlled treatment period, the Sunrise 2 study continued for an additional 6 months of nightly active treatment for continued safety and efficacy assessment. Patients assigned to lemborexant, 5 mg or 10 mg, during the initial period continued on those doses. Those in the placebo group were randomized to either of the 2 lemborexant doses.
Continue to: Safety studies and adverse reactions
Safety studies and adverse reactions
Potential medication effects on middle-of-the-night and next-morning postural stability (body sway measured with an ataxiameter) and cognitive performance, as well as middle-of-the-night auditory awakening threshold, were assessed in a randomized, 4-way crossover study of 56 healthy older adults (women age ≥55 [77.8%], men age ≥65) given a single bedtime dose of placebo, lemborexant, 5 mg, lemborexant, 10 mg, and zolpidem extended-release, 6.25 mg, on separate nights.11 The results were compared with data from a baseline night with the same measures performed prior to the randomization. The middle-of-the-night assessments were done approximately 4 hours after the dose and the next-morning measures were done after 8 hours in bed. The auditory threshold analysis showed no significant differences among the 4 study nights. Compared with placebo, the middle-of-the-night postural stability was significantly worse for both lemborexant doses and zolpidem; however, the zolpidem effect was significantly worse than with either lemborexant dose. The next-morning postural stability measures showed no significant difference from placebo for the lemborexant doses, but zolpidem continued to show a significantly worsened result. The cognitive performance assessment battery provided 4 domain factor scores (power of attention, continuity of attention, quality of memory, and speed of memory retrieval). The middle-of-the-night battery showed no significant difference between lemborexant, 5 mg, and placebo in any domain, while both lemborexant, 10 mg, and zolpidem showed worse performance on some of the attention and/or memory tests. The next-morning cognitive assessment revealed no significant differences from placebo for the treatments.
Respiratory safety was examined in a placebo-controlled, 2-period crossover study of 38 patients diagnosed with mild obstructive sleep apnea who received lemborexant, 10 mg, or placebo nightly during each 8-day period.12 Neither the apnea-hypopnea index nor the mean oxygen saturation during the lemborexant nights were significantly different from the placebo nights.
The most common adverse reaction during the month-long Sunrise 1 study and the first 30 days of the Sunrise 2 study was somnolence or fatigue, which occurred in 1% receiving placebo, 7% receiving lemborexant, 5 mg, and 10% receiving lemborexant, 10 mg. Headache was reported by 3.5% receiving placebo, 5.9% receiving lemborexant, 5 mg, and 4.5% receiving lemborexant, 10 mg. Nightmare or abnormal dreams occurred with 0.9% receiving placebo, 0.9% receiving lemborexant, 5 mg, and 2.2% receiving lemborexant, 10 mg.1
Unique clinical issues
Because investigations of individuals who abused sedatives for recreational purposes showed lemborexant had a likeability rating similar to zolpidem and significantly greater than placebo, the US Drug Enforcement Agency has categorized lemborexant as a Schedule IV controlled substance. Research has not shown evidence of physical dependence or withdrawal signs or symptoms upon discontinuation of lemborexant.1
Contraindications
Narcolepsy is the only contraindication to the use of lemborexant.1 Narcolepsy is associated with a decrease in the orexin-producing neurons in the hypothalamus, presumably causing the excessive sleepiness, sleep paralysis, hypnagogic hallucinations, and cataplexy characteristic of the disorder. Hypothetically, an orexin antagonist medication could exacerbate these symptoms.
Continue to: Dosing
Dosing
Lemborexant should be taken no more than once per night immediately before going to bed and with at least 7 hours remaining before the planned time of awakening.1 The recommended starting dose is 5 mg. The dosage may be increased to a maximum of 10 mg if the initial dose is well tolerated but insufficiently effective. Patients with moderate hepatic impairment or who are concomitantly taking weak CYP3A inhibitors should receive a maximum of 5 mg once nightly. Lemborexant should be avoided in patients with severe hepatic impairment and in those taking moderate or strong CYP3A inhibitors or inducers.
Orexin receptor antagonists do not share cross-tolerance with other hypnotics; this should be taken into consideration when switching to lemborexant. Abruptly stopping a benzodiazepine receptor agonist hypnotic may lead to rebound insomnia and thus may confound the interpretation of the clinical response when starting lemborexant.
Patients prescribed lemborexant should be educated about possible impairment in alertness and motor coordination, especially with the 10-mg dose, which may affect next-morning driving in sensitive individuals.13 Caution is advised with doses >5 mg in patients age ≥65 due to possible somnolence and a higher risk of falls.1
Bottom Line
Lemborexant is a dual orexin receptor antagonist indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. It promotes sleep by suppressing the wake drive supported by the orexin system. In randomized, placebo-controlled trials, lemborexant demonstrated objective and subjective evidence of clinically significant benefits for sleep onset and sleep maintenance.
Related Resource
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
Drug Brand Names
Bupropion • Wellbutrin
Doxepin • Silenor
Eszopiclone • Lunesta
Lemborexant • Dayvigo
Methadone • Methadose, Dolophine
Quazepam • Doral
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien, Intermezzo
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.
1. Dayvigo [package insert]. Woodcliff Lake, NJ: Eisai Inc.; 2020.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
4. Neubauer DN, Pandi-Perumal SR, Spence DW, et al. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672.
5. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
6. Mignot E. Sleep, sleep disorders and hypocretin (orexin). Sleep Med. 2004;5(suppl 1):S2-S8.
7. Boss C, Brisbare-Roch C, Jenck F, et al. Orexin receptor antagonism: a new principle in neuroscience. Chimia. 2008;62:974-979.
8. Landry I, Nakai K, Ferry J, et al. Pharmacokinetics, pharmacodynamics, and safety of the dual orexin receptor antagonist lemborexant: findings from single-dose and multiple-ascending-dose phase 1 studies in healthy adults. Clin Pharmacol Drug Dev. 2020. doi: 10.1002/cpdd.817.
9. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254.
10. Karppa M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43(9):zsaa123. doi: 10.1093/sleep/zsaa123.
11. Murphy P, Kumar D, Zammit G, et al. Safety of lemborexant versus placebo and zolpidem: effects on auditory awakening threshold, postural stability, and cognitive performance in healthy older participants in the middle of the night and upon morning awakening. J Clin Sleep Med. 2020;16(5):765-773.
12. Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: a randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020:e13021. doi: 10.1111/jsr.13021.
13. Vermeeren A, Jongen S, Murphy P, et al. On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4):10.1093/sleep/zsy260. doi: 10.1093/sleep/zsy260.