User login
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
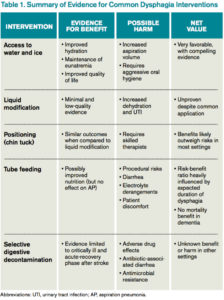
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.