User login
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
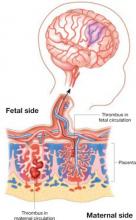
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
The authors report no financial relationships relevant to this article.
Thrombosis is hypothesized to be the more common mechanism underlying cerebral palsy in many cases of maternal or fetal thrombophilia; for that reason, understanding the impact of maternal and fetal thrombophilia on pregnancy outcome is of paramount importance when counseling patients.
Is a maternal and fetal thrombophilia work-up needed in women who give birth to a term infant with cerebral palsy? Prospective studies are needed to evaluate whether that is the case. In this article, we review the literature on fetal thrombophilia and its role in explaining some cases of perinatal stroke that lead, ultimately, to cerebral palsy.
The several causes of cerebral palsy
Cerebral palsy is the most common chronic motor disability of childhood. Approximately 2 to 2.5 of every 1,000 children are given a diagnosis of this disorder every year.1,2 The condition appears early in life; it is not the result of recognized progressive disease.1 Risk factors for cerebral palsy are multiple and heterogenous1,3,4-6:
- Prematurity. The risk of developing cerebral palsy correlates inversely with gestational age.7,8 A premature infant who weighs less than 1,500 g at birth has a risk of cerebral palsy that is 20 to 30 times greater than that of a full-term, normal-weight newborn.3,4
- Hypoxia and ischemia. These are the conditions most often implicated as the cause of cerebral palsy. Fetal heart-rate monitoring was introduced in the 1960s in the hope that interventions to prevent hypoxia and ischemia would reduce the incidence of cerebral palsy. But monitoring has not had that effect—most likely, because some cases of cerebral palsy are caused by perinatal stroke.9 In fact, a large, population-based study has demonstrated that potentially asphyxiating obstetrical conditions account for only about 6% of cases of cerebral palsy.6
- Thrombophilia. Several recent studies report an association between fetal thrombophilia and both neonatal stroke and cerebral palsy.10-14 That association provides a possible explanation for adverse pregnancy outcomes that have otherwise been ascribed to events during delivery.15-23 Although thrombophilia is a recognized risk factor for cerebral palsy, the strength of the association has still not been fully investigated. TABLE 1 and TABLE 2 summarize studies that have examined this association. Given the rarity of both inherited thrombophilias and cerebral palsy, however, an enormous number of cases would be required to fully establish a causal relationship.
TABLE 1
Case reports reveal an association
between fetal thrombophilias and cerebral palsy
| Thrombophilias present | |||
|---|---|---|---|
| Study (type) | Cases of CP | Number | Type |
| Harum et al36 (case report) | 1 | 1 | Factor V Leiden |
| Thorarensen et al37 (case report) | 3 | 3 | Factor V Leiden |
| Lynch et al2 (case series) | 8 | 8 | Factor V Leiden |
| Halliday et al38 (case series) | 55 | 5 | Factor V Leiden; prothrombin mutation |
| Smith et al39 (case series) | 38 | 7 | Factor VIIIc |
| Nelson et al40 (case series) | 31 | 20 | Factor V Leiden; protein C deficiency |
TABLE 2
How often is a fetal thrombophilia
the likely underlying cause of cerebral palsy?
| Thrombophilia* | Prevalence of CP† | Odds ratio |
|---|---|---|
| Factor V Leiden | 6.3% | 0.62 (0.37–1.05) |
| Prothrombin gene | 5.2% | 1.11 (0.59–2.06) |
| MTHFR 677 | 54.1% | 1.27 (0.97–1.66) |
| MTHFR 1298 | 39.4% | 1.08 (0.69–1.19) |
| MTHFR 677/1298 | 15.1% | 1.18 (0.82–1.69) |
| * Heterozygous or homozygous | ||
| † Among 354 subjects with thrombophilia studied41 | ||
| Key: MTHFR, methyltetrahydrofolate reductase | ||
“Thrombophilia” describes a spectrum of congenital or acquired coagulation disorders associated with venous and arterial thrombosis.24 These disorders can occur in the mother or in the fetus, or in both concomitantly.
Fetal thrombophilia has a reported incidence of 2.4 to 5.1 cases for every 100,000 births.25 Whereas maternal thrombophilia has a substantially higher incidence, both maternal and fetal thrombophilia can lead to adverse maternal and fetal events.
The incidence of specific inherited fetal thrombophilias is summarized in TABLE 3. Maternal thrombophilia is generally associated with various adverse pregnancy outcomes, particularly cerebral palsy and perinatal stroke.9,26
TABLE 3
Inherited thrombophilias among the general population
| Study | Number | Factor V Leiden | Protein gene mutation | MTHFR |
|---|---|---|---|---|
| Gibson et al41 (2003) | 708 | 9.8% | 4.7% | 15.1%* |
| Dizon-Townson et al42 (2005) | 4,033 | 3.0% | Not reported | Not reported |
| Infante-Rivard et al43 (2002) | 472 | 3.3% | 1.3% | 43% to 49% |
| Stanley-Christian et al44 (2005) | 14 | 0 | 0 | 0 |
| Currie et al45 (2002) | 46 | 13.0% | Not reported | Not reported |
| Livingston et al46 (2001) | 92 | 0 | 2% | 4% |
| Schlembach et al47 (2003) | 28 | 4.0% | 2% | Not reported |
| Dizon-Townson et al48 (1997) | 130 | 8.6% | Not reported | Not reported |
| * Heterozygous and homozygous carriers of MTHFR C677T and A1298C | ||||
| Key: MTHFR, methyltetrahydrofolate reductase | ||||
Thrombophilia leads to thrombosis at the maternal or fetal interface (FIGURE):
- When thrombosis occurs on the maternal side, the consequence may be severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss.27-29
- Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain.30 As a result, the newborn can sustain a catastrophic event such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.25
Thrombophilia can lead to thrombosis at the maternal or the fetal interface
Thrombosis on the maternal side may lead to severe preeclampsia, intrauterine growth restriction, abruptio placenta, or fetal loss. Thrombosis on the fetal side can be a source of emboli that bypass hepatic and pulmonary circulation and travel to the fetal brain and cause a catastrophic event, such as perinatal arterial stroke via arterial thrombosis, cerebral sinus venous thrombosis, or renal vein thrombosis.
Perinatal and neonatal stroke
Perinatal stroke is defined as a cerebrovascular event that occurs between 28 weeks of gestation and 28 days of postnatal age.30 Incidence is approximately 17 to 93 cases for every 100,000 live births.9
Neonatal stroke occurs in approximately 1 of every 4,000 live births.30 In addition, 1 in every 2,300 to 4,000 newborns is given a diagnosis of ischemic stroke in the nursery.9
Stroke and cerebral palsy
Arterial ischemic stroke in the newborn accounts for 50% to 70% of cases of congenital hemiplegic cerebral palsy.11 Factor V Leiden mutation, prothrombin gene mutation, and a deficiency of protein C, protein S, and antithrombin III have, taken together in two studies, been identified in more than 50% of cerebral ischemic strokes.31,32 In addition to these thrombophilias, important risk factors for perinatal and neonatal stroke include:
- thrombosis in placental villi or vessels
- infection
- use of an intravascular catheter.33
The mechanism that underlies perinatal stroke is a thromboembolic event that originates from either an intracranial or extracranial vessel, the heart, or the placenta.10 A recent meta-analysis by Haywood and colleagues found a statistically significant correlation between protein C deficiency, MTHFR C677T (methyltetrahydrofolate reductase), and the first occurrence of arterial ischemic stroke in a pediatric population.34 Associations between specific thrombophilias and perinatal stroke, as well as pediatric stroke, have been demonstrated (TABLE 4), but we want to emphasize that the absolute risks in these populations are very small.34,35 In addition, the infrequency of these thrombophilias in the general population (TABLE 3) means that their positive predictive value is extremely low.
TABLE 4
Fetal thrombophilia is detected in as many as two thirds of study cases of perinatal and neonatal stroke
| Type of thrombophilia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Infants | Thrombophilia | FVL | APCR | ACA | AT | PC | PS |
| Golomb et al31 | 22 | 14 (63%) * | 1 * | 3 * | 12 * | 0 | 0 | 0 |
| Bonduel et al32 | 30 | 9 (30%) † | n/a | n/a | n/a | 2 | 1 | 2 |
| deVeber et al49 | 92 | 35 (38%) ‡ | 0 | 6 | 23‡ | 10‡ | 6‡ | 3‡ |
| Mercuri et al50 | 24 | 10 (42%) | 5 | n/a | n/a | 0 | 0 | 0 |
| Günther et al35 | 91 | 62 (68%) | 17 | n/a | 3 | 0 | 6 | 0 |
| Govaert et al51 | 40 | 3 (8%) | 3 | n/a | n/a | n/a | n/a | n/a |
| * FVL, APCR, and ACA diagnoses overlapped. | ||||||||
| † Three patients had anticardiolipin antibody and plasminogen deficiency. | ||||||||
| ‡ Of 35 children, 21 had multiple abnormalities (combined coagulation deficiencies). | ||||||||
| Key: ACA, anticardiolipin antibody; APCR, activated protein C resistance; AT, antithrombin deficiency; FVL, factor V Leiden; PC, protein C deficiency; PS, protein S deficiency; n/a, not available or not studied. | ||||||||
Brain injury
The brain is the largest and most vulnerable fetal organ susceptible to thrombi that are formed either in the placenta or elsewhere.16 A review of cases of cerebral palsy has revealed a pathologic finding, fetal thrombotic vasculopathy (FTV), that has been associated with brain injury.16 Arias and colleagues17 and Kraus18 have observed a correlation among cerebral palsy, a thrombophilic state, and FTV.
Furthermore, Redline found that the presence of severe fetal vascular lesions correlated highly with neurologic impairment and cerebral palsy.19
What is the take-home message?
Regrettably for patients and their offspring, evidence about the relationship between thrombophilia and an adverse neurologic outcome is insufficiently strong to offer much in the way of definitive recommendations for the obstetrician.
We can, however, make some tentative recommendations on management:
Consider screening. When cerebral palsy occurs in association with perinatal stroke, fetal and maternal screening for thrombophilia can be performed.34 The recommended thrombophilia panel comprises tests for:
- factor V Leiden
- prothrombin G20210
- anticardiolipin antibody
- MTHFR mutation.10
Family screening has also been suggested in cases of 1) multiple prothrombotic risk factors in an affected newborn and 2) a positive family history.9
The cost-effectiveness of screening for thrombophilia has not been evaluated in prospective studies, because the positive predictive value of such screening is extremely low.
Consider offering prophylaxis, with cautions. A mother whose baby has been given a diagnosis of thrombophilia and fetal or neonatal stroke can be offered thromboprophylaxis (heparin and aspirin) during any subsequent pregnancy. The usefulness of this intervention has not been well studied and is based solely on expert opinion, however, so it is imperative to counsel patients on the risks and benefits of prophylactic therapy beforehand.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.
1. American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington DC: The American College of Obstetricians and Gynecologists; September 2003.
2. Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovascular disorders in children with the factor V Leiden mutation. J Child Neurol. 2001;16:735-744.
3. Gibson CS, MacLennan AH, Goldwater PN, Dekker GA. Antenatal causes of cerebral palsy: associations between inherited thrombophilias, viral and bacterial infection, and inherited susceptibility to infection. Obstet Gynecol Surv. 2003;58:209-220.
4. Ramin SM, Gilstrap LC. Other factors/conditions associated with cerebral palsy. Semin Perinatol. 2000;24:196-199.
5. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613-618.
6. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507-513.
7. Himmelman K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287-294.
8. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220-1225.
9. Nelson KB. Thrombophilias, Thrombosis and Outcome in Pregnancy, Mother, and Child Symposium. Society of Maternal– Fetal Medicine 26th Annual Meeting. Miami Beach, Fla; 2006.
10. Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150-158.
11. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723-729.
12. Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96-97.
13. Fattal-Valevski A, Kenet G, Kupferminc MJ, et al. Role of thrombophilic risk factors in children with non-stroke cerebral palsy. Thromb Res. 2005;116:133-137.
14. Steiner M, Hodes MZ, Shreve M, Sundberg S, Edson JR. Postoperative stroke in a child with cerebral palsy heterozygous for factor V Leiden. J Pediatr Hematol Oncol. 2000;22:262-264.
15. Kraus FT. Perinatal pathology, the placenta and litigation. Human Pathol. 2003;34:517-521.
16. Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies and cerebral palsy. Hum Pathol. 1999;30:759-769.
17. Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277-286.
18. Kraus FT. Cerebral palsy and thrombi in placental vessels in the fetus: insights from litigation. Hum Pathol. 1997;28:246-248.
19. Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452-457.
20. Kraus FT. Placental thrombi and related problems. Semin Diagn Pathol. 1993;10:275-283.
21. Rayne SC, Kraus FT. Placental thrombi and other vascular lesions: classification, morphology and clinical correlations. Pathol Res Pract. 1993;189:2-17.
22. Grafe MR. The correlation of prenatal brain damage and placental pathology. J Neuropathol Exp Neurol. 1994;53:407-415.
23. Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785-1791.
24. Paidas MJ, Ku DH, Arkel YS. Screening and management of inherited thrombophilias in the setting of adverse pregnancy outcome. Clin Perinatol. 2004;31:783-805.
25. Kenet G, Nowak-Göttl U. Fetal and neonatal thrombophilia. Obstet Gynecol Clin North Am. 2006;33:457-466.
26. Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191:412-414.
27. Stella CL, How HY, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome: controversies in screening and management. Am J Perinatol. 2006;23:499-506.
28. Stella CL, Sibai BM. Thrombophilia and adverse maternal–perinatal outcome. Clin Obstet Gynecol. 2006;49:850-860.
29. Sibai BM. Thrombophilia and severe preeclampsia: time to screen and treat in future pregnancies? Hypertension. 2005;46:1252-1253.
30. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurologic Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116-123.
31. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol. 2001;50:163-168.
32. Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with arterial ischemic stroke and sinovenous thrombosis. Arch Neurol. 1999;56:967-971.
33. Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;358:374.-
34. Haywood S, Leisner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402-405.
35. Günther G, Junker R, Sträter R, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke. 2000;31:2437-2441.
36. Harum KH, Hoon AH, Jr, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol. 1999;41:777-780.
37. Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann Neurol. 1997;42:372-375.
38. Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and factor V Leiden mutation. J Med Genet. 2000;37:787-789.
39. Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia a factor in the development of hemiplegic cerebral palsy? Dev Med Child Neurol. 2001;43:724-730.
40. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665-675.
41. Gibson CS, MacLennan A, Hague B, et al. Fetal thrombophilic polymorphisms are not a risk factor for cerebral palsy. Am J Obstet Gynecol. 2003;189 Suppl 1:S75.-
42. Dizon-Townson D, Miller C, Sibai BM, et al. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517-524.
43. Infante-Rivard C, Rivard GE, Yotov WV, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19-25.
44. Stanley-Christian H, Ghidini A, Sacher R, Shemirani M. Fetal genotype for specific inherited thrombophilia is not associated with severe preeclampsia. J Soc Gynecol Investig. 2005;12:198-201.
45. Currie L, Peek M, McNiven M, Prosser I, Mansour J, Ridgway J. Is there an increased maternal–infant prevalence of Factor V Leiden in association with severe pre-eclampsia? BJOG. 2002;109:191-196.
46. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, Sibai BM. Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol. 2001;185:153-157.
47. Schlembach D, Beinder E, Zingsem J, Wunsiedler U, Beckmann MW, Fischer T. Association of maternal and/or fetal factor V Leiden and G20210A prothrombin mutation with HELLP syndrome and intrauterine growth restriction. Clin Sci (Lond). 2003;105:279-285.
48. Dizon-Townson DS, Meline L, Nelson LM, Varner M, Ward K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am J Obstet Gynecol. 1997;177:402-405.
49. deVeber G, Monagle P, Chan A, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998;55:1539-1543.
50. Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics. 2001;107:1400-1404.
51. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A, Vanzieleghem B. Perinatal cortical infarction within middle cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed. 2000;82:F59-F63.