User login
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
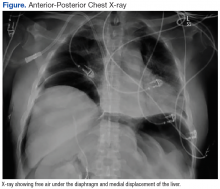
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
Tension pneumoperitoneum (TPP), also known as hyperacute abdominal compartment syndrome or abdominal tamponade, is a rare condition most commonly associated with gastrointestinal (GI) perforation during endoscopy and iatrogenic insufflation of gas into the peritoneal cavity.1 Other reported causes of TPP include gastric rupture after cardiopulmonary resuscitation, barotrauma during scuba diving, positive pressure ventilation through pleural-peritoneal channels, and spontaneous TPP of uncertain mechanism.1-4
Case Presentation
A 76-year-old male with a history of ischemic cardiomyopathy, hypertension, and diabetes mellitus presented to the VA Puget Sound Health Care System in Seattle, Washington emergency department with painless jaundice, hematemesis, melena, and acute renal failure. On esophagogastroduodenoscopy (EGD), he was found to have an ulcer on the posterior wall of the duodenal bulb. The ulcer was coagulated and injected with epinephrine. The patient’s subsequent hospital course was complicated by worsening liver function, the need for renal replacement therapy, and recurrence of upper GI bleeding that required a transcatheter embolization of 2 separate superior pancreaticoduodenal arteries (SPDA) and the inferior pancreaticoduodenal artery (IPDA).
Once clinically stable, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to evaluate for cholangiocarcinoma. A stricture was discovered in the common hepatic duct, brushings were taken, and a 15 cm, 7 Fr stent was placed in the common hepatic duct. The procedure was performed with an Olympus TJF Type Q180V duodenovideoscope (Tokyo, Japan) with an external diameter of 13.7 mm. The patient became hypotensive during the procedure and was treated with phenylephrine and ephedrine boluses. There was no endoscopic evidence of bleeding or bowel trauma.
After completion of the procedure, in the recovery area the patient became severely hypotensive and unresponsive. The physical examination was noteworthy for gross abdominal distention. Arterial blood gas analysis revealed severe metabolic and respiratory acidosis. Chest radiography demonstrated massive pneumoperitoneum, low lung volumes, and diaphragmatic compression (Figure).
A diagnosis of tension pneumoperitoneum was made, and as the patient was transported to the operating room he became bradycardic without a pulse, requiring initiation of cardiopulmonary resuscitation. The abdomen was decompressed with a 14-gauge needle, followed by insertion of a laparoscopic trocar as a decompressive maneuver. This procedure resulted in return of spontaneous circulation.
An exploratory laparotomy was performed, and a massive rush of air was noted on opening the peritoneum. A pinhole perforation of the anterior wall of the second portion of the duodenum was found along with large-volume bilious ascites. This perforation was repaired with a Graham patch, and the patient was taken to the intensive care unit. Postoperatively, the patient developed disseminated intravascular coagulation, shock liver, and acute respiratory distress syndrome, expiring 10 days later from sequelae of multiorgan failure.
Discussion
In relation to upper GI endoscopic procedures, TPP has been reported after diagnostic EGD, endoscopic sphincterotomy, and submucosal tumor dissection.5-7 During these interventions, clinically apparent or overt iatrogenic perforations can occur either in the stomach or duodenum. These perforations may function as one-way valves that cause massive air accumulation and marked elevation of the diaphragm, which severely decreases lung volumes, pulmonary compliance, and limits gas exchange. Hemodynamically, compression of the inferior vena cava restricts venous return to the heart, resulting in decreased cardiac output.8
Patients with TPP present in acute distress with dyspnea, abdominal pain, and shock. On physical examination the abdomen is markedly distended, tympanic, and rigid. Rectal prolapse and subcutaneous emphysema also may be present.9 Roentographic features of TPP include findings of intraperitoneal air with elevation of the diaphragm, medial displacement of the liver (saddlebag sign), and juxtaposition of air in visceral interfaces, making intra-abdominal structures (spleen and gallbladder) appear more discrete.10 Abdominal computer tomography may show massive pneumoperitoneum with bowel loop compression and centralization of abdominal organs.4
Treatment strategies include emergent decompression either with percutaneous catheter insertion or abdominal drain placement followed by a definitive surgical repair. As with management of tension pneumothorax, treatment should not be delayed while awaiting confirmatory radiologic studies.9 When percutaneous needle decompression is undertaken, it is preferable to use a large bore (14-gauge venous catheter) and to advance a catheter over a needle to minimize the risk of visceral injury with egress of air and return of abdominal organs to their normal anatomical positions. The needle should be inserted directly above or below the umbilicus or in the left or right lower quadrants to avoid solid organ (ie, liver or spleen) injury.
Etiologic possibilities for the duodenal perforation in this case include mechanical trauma from the endoscope and duodenal tissue infarction after embolization of a bleeding duodenal ulcer. The duodenum and pancreatic head have a dual blood supply from the SPDA, a branch of the gastroduodenal artery, and the IPDA, a branch of the superior mesenteric artery.11 After failed endoscopic management of persistent duodenal hemorrhage, the patient underwent synchronous embolization of 2 separate SPDAs and the IPDA. This might have put the first 2 segments of the duodenum at risk for ischemic damage and caused it to perforate at some point during the patient’s hospitalization (as evidenced by the bilious ascitis) or rendered them vulnerable to perforation from intraluminal insufflation during endoscopy.12
During the laparotomy, a pinhole-sized perforation was noted in the anterior wall of the second part of the duodenum, distinct form the duodenal ulcer present on the posterior wall. This perforation likely provided a pathway for the intraluminal gas to escape into the peritoneal cavity, culminating in abdominal tamponade, cardiopulmonary deterioration, and arrest. Needle decompression of the abdominal cavity provided an effective, though temporizing relief of this pressure, enabling return of spontaneous circulation.
This case highlights the need for a high index of suspicion for TPP in a patient with cardiopulmonary compromise and abdominal distension after upper GI endoscopic procedures even in the absence of identifiable perforations. Close coordination among gastroenterologists, anesthesiologists, and surgeons is key in prevention, recognition, and management of this rare but catastrophic complication.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.
1. Bunni J, Bryson PJ, Higgs SM. Abdominal compartment syndrome caused by tension pneumoperitoneum in a scuba diver. Ann R Coll Surg Engl. 2012;94(8):e237-e239.
2. Cameron PA, Rosengarten PL, Johnson WR, Dziukas L. Tension pneumoperitoneum after cardiopulmonary resuscitation. Med J Aust. 1991;155(1):44-47.
3. Burdett-Smith P, Jaffey L. Tension pneumoperitoneum. J Accid Emerg Med. 1996;13(3):220-221.
4. Joshi D, Ganai B. Radiological features of tension pneumoperitoneum. BMJ Case Rep. 2015;2015.
5. Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94(3):845-847.
6. Iyilikci L, Akarsu M, Duran E, et al. Duodenal perforation and bilateral tension pneumothorax following endoscopic sphincterotomy. J Anesth. 2009;23(1):164-165.
7. Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(suppl 2):E152.
8. Deenichin GP. Abdominal compartment syndrome. Surg Today. 2008;38(1):5-19.
9. Chiapponi C, Stocker U, Korner M, et al. Emergency percutaneous needle decompression for tension pneumoperitoneum. BMC Gastroenterol. 2011;11:48.
10. Lin BW, Thanassi W. Tension pneumoperitoneum. J Emerg Med. 2010;38(1):57-59.
11. Bell SD, Lau KY, Sniderman KW. Synchronous embolization of the gastroduodenal artery and the inferior pancreaticoduodenal artery in patients with massive duodenal hemorrhage. J Vasc Interv Radiol. 1995;6(4):531-536.
12. Wang YL, Cheng YS, Liu LZ, He ZH, Ding KH. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18(34):4765-4770.