User login
Diet plays an important role in the pathophysiology of irritable bowel syndrome (IBS) and is an effective tool in managing this disorder. This includes a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs).
These indigestible and poorly absorbed short-chain carbohydrates trigger IBS symptoms and are thought to exert their effects by increasing osmotic pressure in the lumen of the intestine and by providing a substrate for bacterial fermentation with consequent gas production.1 The gas causes abdominal distention, and the change in pressure in the lumen of the large intestine affects the release of serotonin, causing abdominal pain and discomfort.
THE MECHANISMS ARE COMPLICATED
Recent studies have shown that the mechanisms by which FODMAPs exert their effects are more complicated than originally thought.1
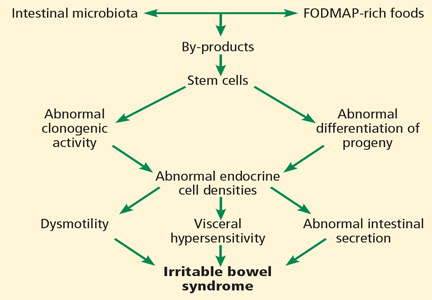
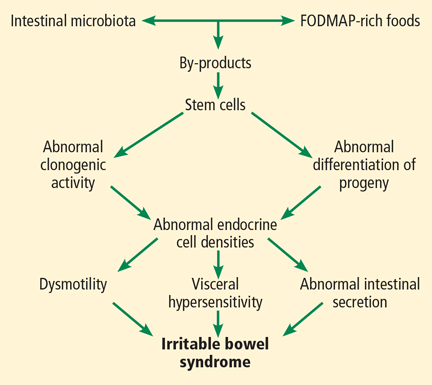
All segments of the gastrointestinal tract contain endocrine cells scattered between the mucosal epithelial cells facing the intestinal lumen.1,2 There are at least 10 types of endocrine cell, and they regulate gastrointestinal motility, secretion, absorption, visceral sensitivity, local immune defense, cell proliferation, and appetite.2–4 Abnormal densities of gastrointestinal endocrine cells have been reported in patients with IBS, which may explain the dysmotility, visceral hypersensitivity, and abnormal intestinal secretion seen in these patients.5
But other factors such as diet, intestinal microbiota, genetics, and low-grade inflammation also play pivotal roles in the pathophysiology of IBS by exerting effects on gastrointestinal endocrine cells. The abnormalities in the gastrointestinal endocrine cells in IBS are thought to be brought about by aberrant differentiation of stem cells into endocrine cells (Figure 1).6
A diet low in FODMAPs appears to induce changes in the intestinal microbiota and gastrointestinal endocrine cells and to reduce IBS symptoms.6
GLUTEN IS IMPLICATED
Another dietary factor in IBS is gluten. Symptoms of IBS and celiac disease overlap: most studies have found that fewer than 5% of patients with celiac disease are misdiagnosed as having IBS based on the symptom criteria for IBS, but some studies report a rate as high as 32%.7 In addition, 38% of patients with celiac disease who consume a gluten-free diet fulfill the symptom-based Rome criteria for IBS.7
The contribution of gluten to IBS does not end with the coexistence of IBS and celiac disease, but also includes the newly debated diagnosis of nonceliac gluten sensitivity, characterized by gastrointestinal symptoms (abdominal pain, diarrhea, constipation, nausea, and vomiting) and other symptoms (headache, musculoskeletal pain, “brain fog,” fatigue, and depression) that are similar to those of IBS. Symptoms are triggered by the ingestion of wheat products, are improved after wheat products are removed from the diet, and relapse after a wheat challenge.7
Nonceliac gluten sensitivity is often perceivable by patients, resulting in self-diagnosis and self-treatment.4 However, it is not clear whether it is gluten or the fructans and galactans in wheat that are responsible for triggering their symptoms.7
DIETARY GUIDANCE IS NEEDED
A low-FODMAP diet with small amounts of insoluble dietary fiber improves symptoms and quality of life in patients with IBS, but dietary guidance is critical and should be personalized because patients differ in how they tolerate foods rich in FODMAPs, probably owing to differing intestinal microbiota among individuals.3,8 Intake of probiotics increases tolerance of FODMAP-rich foods and should also be recommended.3,8,9 Because of the rigorous restrictions of the low-FODMAP diet, patients who receive personalized guidance are more inclined to adhere to the diet and to avoid vitamin and mineral deficiencies.9
- El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J 2015; 14:36–46.
- El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012; 4:2783–2800.
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. 1st ed. New York, NY: Nova Science Publishers, Inc.; 2014.
- El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (review). Int J Mol Med 2012; 29:723–731.
- El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol 2014; 20:384–400.
- El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 2015; 21:7621–7636.
- El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr J 2015; 14:92–99.
- El-Salhy M, Lillebo E, Reinemo A, Salmelid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotic administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterology Insights 2010; 2:21–26. doi: http://dx.doi.org/10.4081/gi.2010.e6.
- Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep 2012; 5:1382–1390.
Diet plays an important role in the pathophysiology of irritable bowel syndrome (IBS) and is an effective tool in managing this disorder. This includes a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs).
These indigestible and poorly absorbed short-chain carbohydrates trigger IBS symptoms and are thought to exert their effects by increasing osmotic pressure in the lumen of the intestine and by providing a substrate for bacterial fermentation with consequent gas production.1 The gas causes abdominal distention, and the change in pressure in the lumen of the large intestine affects the release of serotonin, causing abdominal pain and discomfort.
THE MECHANISMS ARE COMPLICATED
Recent studies have shown that the mechanisms by which FODMAPs exert their effects are more complicated than originally thought.1
All segments of the gastrointestinal tract contain endocrine cells scattered between the mucosal epithelial cells facing the intestinal lumen.1,2 There are at least 10 types of endocrine cell, and they regulate gastrointestinal motility, secretion, absorption, visceral sensitivity, local immune defense, cell proliferation, and appetite.2–4 Abnormal densities of gastrointestinal endocrine cells have been reported in patients with IBS, which may explain the dysmotility, visceral hypersensitivity, and abnormal intestinal secretion seen in these patients.5
But other factors such as diet, intestinal microbiota, genetics, and low-grade inflammation also play pivotal roles in the pathophysiology of IBS by exerting effects on gastrointestinal endocrine cells. The abnormalities in the gastrointestinal endocrine cells in IBS are thought to be brought about by aberrant differentiation of stem cells into endocrine cells (Figure 1).6
A diet low in FODMAPs appears to induce changes in the intestinal microbiota and gastrointestinal endocrine cells and to reduce IBS symptoms.6

GLUTEN IS IMPLICATED
Another dietary factor in IBS is gluten. Symptoms of IBS and celiac disease overlap: most studies have found that fewer than 5% of patients with celiac disease are misdiagnosed as having IBS based on the symptom criteria for IBS, but some studies report a rate as high as 32%.7 In addition, 38% of patients with celiac disease who consume a gluten-free diet fulfill the symptom-based Rome criteria for IBS.7
The contribution of gluten to IBS does not end with the coexistence of IBS and celiac disease, but also includes the newly debated diagnosis of nonceliac gluten sensitivity, characterized by gastrointestinal symptoms (abdominal pain, diarrhea, constipation, nausea, and vomiting) and other symptoms (headache, musculoskeletal pain, “brain fog,” fatigue, and depression) that are similar to those of IBS. Symptoms are triggered by the ingestion of wheat products, are improved after wheat products are removed from the diet, and relapse after a wheat challenge.7
Nonceliac gluten sensitivity is often perceivable by patients, resulting in self-diagnosis and self-treatment.4 However, it is not clear whether it is gluten or the fructans and galactans in wheat that are responsible for triggering their symptoms.7
DIETARY GUIDANCE IS NEEDED
A low-FODMAP diet with small amounts of insoluble dietary fiber improves symptoms and quality of life in patients with IBS, but dietary guidance is critical and should be personalized because patients differ in how they tolerate foods rich in FODMAPs, probably owing to differing intestinal microbiota among individuals.3,8 Intake of probiotics increases tolerance of FODMAP-rich foods and should also be recommended.3,8,9 Because of the rigorous restrictions of the low-FODMAP diet, patients who receive personalized guidance are more inclined to adhere to the diet and to avoid vitamin and mineral deficiencies.9
Diet plays an important role in the pathophysiology of irritable bowel syndrome (IBS) and is an effective tool in managing this disorder. This includes a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs).
These indigestible and poorly absorbed short-chain carbohydrates trigger IBS symptoms and are thought to exert their effects by increasing osmotic pressure in the lumen of the intestine and by providing a substrate for bacterial fermentation with consequent gas production.1 The gas causes abdominal distention, and the change in pressure in the lumen of the large intestine affects the release of serotonin, causing abdominal pain and discomfort.
THE MECHANISMS ARE COMPLICATED
Recent studies have shown that the mechanisms by which FODMAPs exert their effects are more complicated than originally thought.1
All segments of the gastrointestinal tract contain endocrine cells scattered between the mucosal epithelial cells facing the intestinal lumen.1,2 There are at least 10 types of endocrine cell, and they regulate gastrointestinal motility, secretion, absorption, visceral sensitivity, local immune defense, cell proliferation, and appetite.2–4 Abnormal densities of gastrointestinal endocrine cells have been reported in patients with IBS, which may explain the dysmotility, visceral hypersensitivity, and abnormal intestinal secretion seen in these patients.5
But other factors such as diet, intestinal microbiota, genetics, and low-grade inflammation also play pivotal roles in the pathophysiology of IBS by exerting effects on gastrointestinal endocrine cells. The abnormalities in the gastrointestinal endocrine cells in IBS are thought to be brought about by aberrant differentiation of stem cells into endocrine cells (Figure 1).6
A diet low in FODMAPs appears to induce changes in the intestinal microbiota and gastrointestinal endocrine cells and to reduce IBS symptoms.6

GLUTEN IS IMPLICATED
Another dietary factor in IBS is gluten. Symptoms of IBS and celiac disease overlap: most studies have found that fewer than 5% of patients with celiac disease are misdiagnosed as having IBS based on the symptom criteria for IBS, but some studies report a rate as high as 32%.7 In addition, 38% of patients with celiac disease who consume a gluten-free diet fulfill the symptom-based Rome criteria for IBS.7
The contribution of gluten to IBS does not end with the coexistence of IBS and celiac disease, but also includes the newly debated diagnosis of nonceliac gluten sensitivity, characterized by gastrointestinal symptoms (abdominal pain, diarrhea, constipation, nausea, and vomiting) and other symptoms (headache, musculoskeletal pain, “brain fog,” fatigue, and depression) that are similar to those of IBS. Symptoms are triggered by the ingestion of wheat products, are improved after wheat products are removed from the diet, and relapse after a wheat challenge.7
Nonceliac gluten sensitivity is often perceivable by patients, resulting in self-diagnosis and self-treatment.4 However, it is not clear whether it is gluten or the fructans and galactans in wheat that are responsible for triggering their symptoms.7
DIETARY GUIDANCE IS NEEDED
A low-FODMAP diet with small amounts of insoluble dietary fiber improves symptoms and quality of life in patients with IBS, but dietary guidance is critical and should be personalized because patients differ in how they tolerate foods rich in FODMAPs, probably owing to differing intestinal microbiota among individuals.3,8 Intake of probiotics increases tolerance of FODMAP-rich foods and should also be recommended.3,8,9 Because of the rigorous restrictions of the low-FODMAP diet, patients who receive personalized guidance are more inclined to adhere to the diet and to avoid vitamin and mineral deficiencies.9
- El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J 2015; 14:36–46.
- El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012; 4:2783–2800.
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. 1st ed. New York, NY: Nova Science Publishers, Inc.; 2014.
- El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (review). Int J Mol Med 2012; 29:723–731.
- El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol 2014; 20:384–400.
- El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 2015; 21:7621–7636.
- El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr J 2015; 14:92–99.
- El-Salhy M, Lillebo E, Reinemo A, Salmelid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotic administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterology Insights 2010; 2:21–26. doi: http://dx.doi.org/10.4081/gi.2010.e6.
- Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep 2012; 5:1382–1390.
- El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J 2015; 14:36–46.
- El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012; 4:2783–2800.
- El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. 1st ed. New York, NY: Nova Science Publishers, Inc.; 2014.
- El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (review). Int J Mol Med 2012; 29:723–731.
- El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol 2014; 20:384–400.
- El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol 2015; 21:7621–7636.
- El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. The relation between celiac disease, nonceliac gluten sensitivity and irritable bowel syndrome. Nutr J 2015; 14:92–99.
- El-Salhy M, Lillebo E, Reinemo A, Salmelid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotic administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterology Insights 2010; 2:21–26. doi: http://dx.doi.org/10.4081/gi.2010.e6.
- Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep 2012; 5:1382–1390.