User login
- Expect to encounter 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma ever y year.
- There is good evidence for using the American Cancer Society’s ABCDE criteria as a clinical diagnostic test to rule out malignant melanoma (A).
- The revised 7-point checklist has high sensitivity and is therefore useful for ruling out a diagnosis of malignant melanoma. However, its low specificity yields many false-positive results (B).
- The gold standard for diagnosis of skin malignancies is a tissue biopsy. If any doubt exists about the diagnosis, a biopsy should be performed (A).
The American Cancer Society’s ABCDE criteria and the revised 7-point checklist are the most reliable means of detecting or ruling out malignant melanoma. Each has its strengths and weaknesses, a knowledge of which will increase the accuracy of assessment and minimize chances of misdiagnosis.
In addition to these 2 clinical prediction rules, we examine the evidence on physician’s global assessment of nonmelanoma skin cancers and review the risk factors for the major types of skin cancer. As a result of a comprehensive evidence-based review on the incidence, risk factors, and diagnosis of skin malignancies, we present an algorithm for evaluating skin lesions.
Impact of skin cancer
The incidence of malignant melanoma has increased from 1 in 1500 in 1930 to 1 in 75 for the year 2000.1 Although it is the rarest skin cancer (1% of skin malignancies), it is also the deadliest, accounting for 60% of skin cancer deaths.2
Nonmelanoma skin cancers, which include squamous cell cancers and basal cell cancers, account for one third of all cancers in the United States. Approximately 1 million cases were diagnosed in 1999.3 Deaths from nonmelanoma skin cancers are in steady decline, and the overall 5-year survival rate is high (over 95%).4 Recurrent nonmelanoma skin cancer, however, carries a very poor prognosis, with only a 50% cure rate.5
Treatment of nonmelanoma skin cancer costs over $500 million yearly in the US.4
Primary care physicians help improve prognosis
More persons visit primary care physicians (38.2%) than dermatologists (29.9%) for evaluation of suspicious skin lesions.6 Such lesions are usually benign, but a malignancy must be excluded. A primary care physician can expect to diagnose 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma every year, according to population-based studies.4
Primary care practitioners contribute to a more favorable prognosis. For each additional family physician per 10,000 population, the chances of diagnosing malignant melanoma earlier increase significantly (odds ratio= 1.21, 95% confidence interval, 1.09–1.33, P<.001).7
Primary care physicians who diagnose non-melanoma skin cancers can select therapies that offer maximum efficacy and cost-effectiveness.
Differential diagnosis
According to a study of 1215 biopsies conducted in a primary care population, over 80% of biopsied lesions were benign and included nevi, seborrheic keratoses, cysts, dermatofibromas, fibrous histiocytomas, and polyps or skin tags. Pre-malignant lesions (including actinic keratoses and lentigo maligna) represented 7% of the total. Thirteen percent were malignancies: basal cell carcinomas (73%), followed by squamous cell carcinomas (14%), and malignant melanomas (12%). One metastatic adenomacarcinoma was included in the series (1%) (level of evidence [LOE]: 4).8
The differential diagnosis for basal cell carcinoma includes superficial basal cell carcinoma, pigmented basal cell carcinoma, infiltrating basal cell carcinoma, tricoepithelioma, keloid, molluscum contagiosum, and dermatofibromas.
For squamous cell cancer, the differential includes squamous cell carcinoma, keratoacanthoma, eczema and atopic dermatitis, contact dermatitis, psoriasis, and seborrheic dermatitis.
The differential diagnosis for malignant melanoma includes seborrheic keratosis, traumatized or irritated nevus, pigmented basal cell carcinoma, lentigo, blue nevus, angiokeratoma, traumatic hematoma, venous lake, hemangioma, dermatofibroma, and pigmented actinic keratosis.
Using the history and physical examination
Nearly 70% of melanomas are discovered by patients or their family (LOE: 4).9 Patients may express concern about changed size or appearance of a lesion; associated pain, pruritis, ulceration, or bleeding; location in a cosmetically sensitive area; or worry voiced by a family member. Additionally, a patient may have a family or personal history of skin malignancy, history of skin biopsy, or predisposition to sunburns.
Nurses and physicians identify lesions before a patient does approximately one quarter of the time while examining a patient for an unrelated condition or as part of a comprehensive work-up (LOE: 4).9
Types of skin malignancies
See Photo Rounds, page 219, for images of many types of skin cancer.
Basal cell carcinoma
The patterns of basal cell carcinoma are nodular, superficial, micronodular, infiltrative, morpheaform, and mixed.10 They may be pigmented and are sometimes misdiagnosed as melanoma.11 However, most basal cell carcinomas are typical in appearance and easily diagnosed by visual and tactile inspection.
The most common nodular type is a smooth, skin-colored, indurated, dome-shaped papule with a rolled edge. Other attributes include a pearly appearance, overlying telangiectatic vessels, and a history of bleeding with minor trauma.7,11
Superficial basal cell carcinoma is similar to dermatitis but more often has distinct borders and a bright pink appearance.11 If in doubt about the diagnosis, obtain a tissue sample for pathology.
Squamous cell carcinoma
Squamous cell carcinoma most often is a small, firm, hyperkaratotic nodule sitting atop an inflamed base. It may also be skin-colored and smooth. The history can include itching, pain, and nonhealing after minor trauma.7,11,12 As with basal cell carcinoma, diagnosis is made by tissue pathology.
Malignant melanoma
Malignant melanoma usually appears as a changing or unusual mole with haphazard color variegation, including combinations of brown, black, blue, gray, white, and (rarely) pink. Most melanomas are larger than 5 mm in diameter at time of diagnosis.13
There are 4 main types of malignant melanoma:
- Superficial spreading melanoma accounts for 50% of cases and occurs more frequently in younger adults.
- Nodular melanoma also occurs in younger adults, representing 20% to 25% of cases.
- Lentigo maligna melanoma occurs in older adults and accounts for only 15% of cases.
- Acral or acral-lentiginous melanomas are the least common form (10% of cases). They appear on the palms, soles, and around the first toenail.14
Risk factors for skin malignancies
Factors conferring the highest relative risk for malignant melanoma include:13
- atypical nevus syndrome with a personal and family history of melanoma
- history of a changing mole
- atypical nevus syndrome with just a family history of melanoma
- age greater than or equal to 15 years
- history of dysplastic moles.
Table 1 provides a list of risk factors that should prompt an annual skin survey (LOE: 5).
For nonmelanoma skin cancers, the strongest risk factors ( Table 2) include Caucasian race; age 55 to 75 years; and male sex.2 There is good evidence that a history of nonmelanoma skin cancer confers a 10-fold risk for recurrence (LOE: 2a).15 A distinct risk factor for squamous cell carcinoma is immunosuppression.2 Table 2 also provides a complete list of risk factors for nonmelanoma skin cancer.
Precursor lesions for nonmelanoma skin cancers include Bowen’s disease and erythroplasia of Queyrat (forms of squamous cell carcinoma in situ that will progress if left untreated). Actinic keratoses are common precursor lesions, but their overall annual rate of malignant transformation is only 1 in 400. In the case of SCC, up to 60% of cancers develop from an existing actinic keratosis.2
TABLE 1
Risk factors for malignant melanoma 13
| Risk factors that should prompt an annual skin survey | RR (LOE)* |
|---|---|
| Atypical nevus syndrome with personal and family history of melanoma | 500 (1b) |
| Changing mole | >400 (4) |
| Atypical nevus syndrome with family history of melanoma | 148 (1b)† |
| Age ≥ 15 | 88 (2c) |
| Dysplastic moles | 7–70 (3b) |
| History of melanoma before age 40 | 23 (2b) |
| Large congenital nevus (≥15 cm) | 17 (2b) |
| Caucasian race | 12 (2b) |
| Lentigo maligna | 10 (2c) |
| Atypical nevi | 7–27 (3b) |
| Regular use of tanning bed before age 30 | 7.7‡ (3b) |
| Multiple nevi | 5–12 (3b) |
| Personal history of melanoma | 5–9 (2b) |
| Immunosupression | 4–8 (2b) |
| Family history (first degree) of melanoma | 3–8 (3b) |
| Nonmelanoma skin cancer | 3–5 (3b) |
| Sun sensitivity or tendency to burn | 2–3 (3b) |
| *See page 239 for a description of levels of evidence | |
| †(95% CI, 40–379) | |
| ‡(95% CI, 1–63.6) | |
| RR, relative risk (compared with person without risk factors); | |
| LOE, level of evidence; | |
| CI, confidence interval | |
TABLE 2
Risk factors for nonmelanoma skin cancer
| Significant risk factors | RR | LOE* |
|---|---|---|
| Caucasian race | 70 | 2c |
| Immunosuppression | 5–20 | 2c |
| Previous nonmelanoma skin cancer | 10 | 2a |
| Age 55–75 | 4–8 | 2c |
| Male sex | 2 | 2c |
| Genetic risk factors associated with nonmelonoma skin cancer 3 | ||
| ||
| Chemical exposure risk factors associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| Environmental factors and medical conditions associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| *See page 239 for a description of levels of evidence | ||
| RR, relative risk (compared with person without risk factors); LOE, level of evidence | ||
Clinical prediction rules for skin malignancies
Malignant melanoma
ABCDE criteria. A useful clinical prediction rule for malignant melanoma is the American Cancer Society’s “ABCDE criteria” (Table 3). This rule was validated in 4 dermatology clinics, studying a total of 1118 lesions, although the studies were not homogenous (strength of recommendation [SOR]: A).16-19 Results of the study are summarized in Table 4. The test is normally considered positive if one or more of the criteria are met; however, as more criteria are met, specificity increases while sensitivity decreases.17-19
For lesions lacking any of the ABCDE criteria, 99.8% are something other than melanoma (using a prevalence of 1% found in the US population) (SOR: A). Use caution, however, as this rule will miss amelanotic melanomas, as well as smaller melanomas that are changing in size or have other features suggestive of malignant melanoma.
Conversely, if one of the criteria is met, there is nearly a 1.5% (positive predictive value) probability it is melanoma. Excisional biopsy of the lesion is indicated if good clinical judgment is used and it cannot be identified with certainty as a typical benign lesion (SOR: A). This test thus guides clinicians when making a decision to biopsy, as well as in choosing a biopsy technique.
The ABCDE criteria establish a risk of malignancy if the lesion is 6 mm in diameter or greater. Some evidence, however, suggests that this value should not be used as an absolute cutoff for diagnosing malignant melanoma. A large retrospective study performed in Australia found that 31% of biopsy-confirmed melanomas were less than 6 mm in diameter (LOE: 2b).20
Revised 7-point checklist. Another potentially useful diagnostic test is the revised 7-point checklist developed in the United Kingdom ( Table 5). This test was found to have a high sensitivity, but low specificity ( Table 4) . Therefore, it has a low false-negative rate, and is useful for ruling out the diagnosis of melanoma when negative. However, the test yields a significant number of false positive results, leading to possibly unnecessary biopsies and increased patient anxiety (SOR: B).16,21,22
Note: the described sensitivities and specificities for both tests apply only to malignant melanomas, and their accuracy decreases when including basal cell and squamous cell carcinomas. Also, the 2 tests were scored differently in some of the validation studies, making attempts to generalize problematic.
Studies of physicians’ global assessments to detect melanomas ( Table 4) vary widely for sensitivity (50% to 97%) but are consistent for specificity (96% to 99%) (SOR: B).23-28 Additionally, some studies have shown higher percentages of correct diagnosis of malignant melanoma among dermatologists compared with nondermatologists, but all these studies (except for a small subset of patients in one) used lesion images rather than patient examinations (SOR: B).23,29-33
More importantly, when the choice of correct treatment was evaluated, no statistically significant difference was found between the two groups. Further prospective cohort trials using patient examinations are needed to evaluate dermatologist performance versus nondermatologist performance.
TABLE 3
American Cancer Society's ABCDE criteria
| The test is considered positive if a lesion exhibits 1 or more of the 5 criteria |
| Assymetry—one half of the lesion not identical to the other |
| Border irregularity—lesion has an uneven or ragged border |
| Color variegation—lesion has more than one color (ie, black, blue, pink, red, or white) |
| Diameter—lesion has a diameter greater than 6 mm |
| Elevation or Enlargement—elevation of lesion above skin surface or enlargement by patient report |
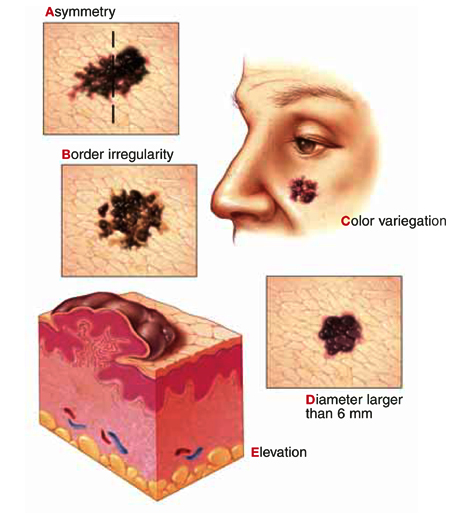 |
TABLE 4
Clinical prediction tests for skin malignancies
| Diagnostic test | Study quality (SOR)* | Sensitivity % (average) | Specificity % (average) | LR+ (1% pretest) | LR– | PV+ | PV– |
|---|---|---|---|---|---|---|---|
| ABCDE criteria (1 criterion positive)16-19 | A | 92–97 (93) | 13–63 (37) | 1.5 | 0.2 | 1.5% | 99.8% |
| Revised 7-point checklist16,21,22 | B | 79–100 (90) | 30–37 (34) | 1.4 | 0.3 | 1.4% | 99.7% |
| Physician global assessments23-28 | B | 50–97 (74) | 96–99 (98) | 37 | 0.3 | 27.2% | 99.7% |
| *See page 239 for a description of strength of recommendation | |||||||
| Note: These calculations are based on simple averages. Statistical homogeneity could not be fully evaluated due to study data limitations. | |||||||
| SOR, strength of recommendation; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, positive predictive value; PV–, negative predictive value | |||||||
TABLE 5
Revised 7-point checklist for assessing risk of melanoma
Suspect melanoma if there are 1 or more major signs:
|
3 or 4 minor signs without a major sign can also indicate a need to biopsy suspicious moles:
|
No validated tool for diagnosis of nonmelanoma skin cancers
A useful diagnostic tool has not yet been validated for nonmelanoma skin cancers. Over 60% of non-melanoma skin cancers occur on the face and neck, and these areas bear careful inspection. Lesions behind the ear, at the medial canthus, and within the nasolabial folds are most easily missed.
How to proceed in assessing lesions
When evaluating skin lesions, remember the gold standard for diagnosis of skin malignancies is a tissue biopsy. If you or your patient has any doubt about the diagnosis, a biopsy should be performed.
To review: Good evidence supports the use the ABCDE criteria or the revised 7-point checklist in determining whether lesions are likely to be malignant melanomas. No similar diagnostic rules exist for basal cell and squamous cell carcinomas. The decision to biopsy these lesions must be based on global assessment and typical characteristics.
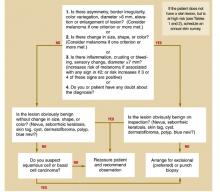
Based on this information, we developed an algorithm for evaluating patients at risk for skin malignancies ( Figure) . The first step is to apply the ABCDE criteria and the revised 7-point checklist to identify or rule out possible malignant melanomas. An excisional biopsy should be performed if either test is positive (and the lesion is not clinically benign), or if you or your patient has any doubt.
If neither of these diagnostic tests yields a positive result, the lesion should be classified as typically benign or as having characteristics suggestive of a squamous cell or basal cell carcinoma.
Lesions that have characteristics of squamous cell or basal cell cancer should be biopsied, and benign lesions can be observed and the patient reassured.
FIGURE
Approach to the patient with a skin lesion
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript. We also gratefully acknowledge Dr. Richard P. Usatine for preparing the accompanying Photo Rounds.
1. Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol 1996;34:839-847.
2. Skin Tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases Philadelphia, Pa: Lippincott-Raven, 1996;342.-
3. Landis SH, Murray T, Bolden S, Wingo PA. Cancer Statistics, 1999. CA Cancer J Clin 1999;49:8-31.
4. Gloster HM, , Jr. Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-96 Summary. Vital Health Stat 1999;13:1-122.
7. Roetzheim RG, Naazneen P, Van Durme DJ. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol 2000;43:211-218.
8. Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. JABFP 1996;9:397-404.
9. Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma: patterns from a population-based survey. J Am Acad Dermatol 1992;26:914-919.
10. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
11. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
12. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
13. Rhodes AR, Weinstock MA, , Jr, Fitzpatrick B, Mihm MC, Jr, Sober AJ. Risk factors for cutaneous melanoma: a practical method of recognizing predisposed individuals. JAMA 1987;258:3146-3154.
14. Austoker J. Melanoma: prevention and early diagnosis. BMJ 1994;308:1682-1686.
15. Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol 2000;136:1524-1530.
16. Healsmith MF, Bourke JF, Osborne JE, Graham-Brown RAC. An evaluation of the revised seven-point checklist for the early diagnosis of cutaneous melanoma. Br J Dermatol 1994;130:48-50.
17. McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions: a comparson of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesions. J Dermatol Surg Oncol 1992;18:22-26.
18. Benelli C, Roscetti E, Dal Pozzo V, Gasparini G, Cavicchini S. The dermoscopic versus the clinical diagnosis of melanoma. Eur J Dermatol 1999;9:470-476.
19. Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology 1998;197:11-17.
20. Shaw HM, McCarthy WH. Small-diameter malignant melanoma: a common diagnosis in New South Wales, Australia. J Am Acad Dermatol 1992;27:679-682.
21. Du Vivier AWP, Williams HC, Brett JV, Higgins EM. How do malignant melanomas present and does this correlate with the seven-point checklist? Clin Exp Dermatol 1991;16:344-347.
22. Higgins EM, Hall P, Todd P, Murthi R, Du Vivier AWP. The application of the seven-point check-list in the assessment of benign pigmented lesions. Clin Exp Dermatol 1992;17:313-315.
23. Whited JD, Grichnik JM. Does this patient have a more or a melanoma? JAMA 2002;279:696-701.
24. Curley RK, Cook MG, Fallowfield ME, Marsden RA. Accuracy in clinically evaluating pigmented lesions. Br Med J 1989;299:16-18.
25. DeCoste SD, Stern RS. Diagnosis and treatment of nevomelanocytic lesion of the skin: a community-based study. Arch Dermatol 1993;129:57-62.
26. Grin CM, Kopf AW, Welkovich B, Bart RS, Levenstein MJ. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol 1990;126:763-766.
27. Koh HK, Caruso A, Gage I, Geller AC, Prout MN, White H, et al. Evaluation of melanoma/skin cancer screening in Massachusetts: preliminary results. Cancer 1990;65:375-379.
28. McMullan FH, Hubener LF. Malignant melanoma: a statistical review of clinical and histological diagnoses. Arch Dermatol 1956;74:618-619.
29. Cassileth BR, Clark WHJ, Lusk EJ, Frederick BE, Thompson CJ, Walsh WP. How well do physicians recognize melanoma and other problem lesions? J Am Acad Dermatol 1986;14:555-560.
30. Gerbert G, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, et al. Primary care physicians as gatekeepers in managed care. Arch Dermatol 1996;132:1030-1038.
31. McGee R, Elwood M, Sneyd MJ, Williams S, Tilyard M. The recognition and management of melanoma and other skin lesions by general practitioners in New Zealand. N Z Med J 1994;107:287-290.
32. Paine SL, Cockburn J, Noy SM, Marks R. Early detection of skin cancer: knowledge, perceptions, and practices of general practitioners in Victoria. Med J Aust 1994;161:188-195.
33. Ramsay DL, Fox AB. The ability of primary care physicians to recognize the common dermatoses. Arch Dermatol 1981;117:620-622.
- Expect to encounter 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma ever y year.
- There is good evidence for using the American Cancer Society’s ABCDE criteria as a clinical diagnostic test to rule out malignant melanoma (A).
- The revised 7-point checklist has high sensitivity and is therefore useful for ruling out a diagnosis of malignant melanoma. However, its low specificity yields many false-positive results (B).
- The gold standard for diagnosis of skin malignancies is a tissue biopsy. If any doubt exists about the diagnosis, a biopsy should be performed (A).
The American Cancer Society’s ABCDE criteria and the revised 7-point checklist are the most reliable means of detecting or ruling out malignant melanoma. Each has its strengths and weaknesses, a knowledge of which will increase the accuracy of assessment and minimize chances of misdiagnosis.
In addition to these 2 clinical prediction rules, we examine the evidence on physician’s global assessment of nonmelanoma skin cancers and review the risk factors for the major types of skin cancer. As a result of a comprehensive evidence-based review on the incidence, risk factors, and diagnosis of skin malignancies, we present an algorithm for evaluating skin lesions.
Impact of skin cancer
The incidence of malignant melanoma has increased from 1 in 1500 in 1930 to 1 in 75 for the year 2000.1 Although it is the rarest skin cancer (1% of skin malignancies), it is also the deadliest, accounting for 60% of skin cancer deaths.2
Nonmelanoma skin cancers, which include squamous cell cancers and basal cell cancers, account for one third of all cancers in the United States. Approximately 1 million cases were diagnosed in 1999.3 Deaths from nonmelanoma skin cancers are in steady decline, and the overall 5-year survival rate is high (over 95%).4 Recurrent nonmelanoma skin cancer, however, carries a very poor prognosis, with only a 50% cure rate.5
Treatment of nonmelanoma skin cancer costs over $500 million yearly in the US.4
Primary care physicians help improve prognosis
More persons visit primary care physicians (38.2%) than dermatologists (29.9%) for evaluation of suspicious skin lesions.6 Such lesions are usually benign, but a malignancy must be excluded. A primary care physician can expect to diagnose 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma every year, according to population-based studies.4
Primary care practitioners contribute to a more favorable prognosis. For each additional family physician per 10,000 population, the chances of diagnosing malignant melanoma earlier increase significantly (odds ratio= 1.21, 95% confidence interval, 1.09–1.33, P<.001).7
Primary care physicians who diagnose non-melanoma skin cancers can select therapies that offer maximum efficacy and cost-effectiveness.
Differential diagnosis
According to a study of 1215 biopsies conducted in a primary care population, over 80% of biopsied lesions were benign and included nevi, seborrheic keratoses, cysts, dermatofibromas, fibrous histiocytomas, and polyps or skin tags. Pre-malignant lesions (including actinic keratoses and lentigo maligna) represented 7% of the total. Thirteen percent were malignancies: basal cell carcinomas (73%), followed by squamous cell carcinomas (14%), and malignant melanomas (12%). One metastatic adenomacarcinoma was included in the series (1%) (level of evidence [LOE]: 4).8
The differential diagnosis for basal cell carcinoma includes superficial basal cell carcinoma, pigmented basal cell carcinoma, infiltrating basal cell carcinoma, tricoepithelioma, keloid, molluscum contagiosum, and dermatofibromas.
For squamous cell cancer, the differential includes squamous cell carcinoma, keratoacanthoma, eczema and atopic dermatitis, contact dermatitis, psoriasis, and seborrheic dermatitis.
The differential diagnosis for malignant melanoma includes seborrheic keratosis, traumatized or irritated nevus, pigmented basal cell carcinoma, lentigo, blue nevus, angiokeratoma, traumatic hematoma, venous lake, hemangioma, dermatofibroma, and pigmented actinic keratosis.
Using the history and physical examination
Nearly 70% of melanomas are discovered by patients or their family (LOE: 4).9 Patients may express concern about changed size or appearance of a lesion; associated pain, pruritis, ulceration, or bleeding; location in a cosmetically sensitive area; or worry voiced by a family member. Additionally, a patient may have a family or personal history of skin malignancy, history of skin biopsy, or predisposition to sunburns.
Nurses and physicians identify lesions before a patient does approximately one quarter of the time while examining a patient for an unrelated condition or as part of a comprehensive work-up (LOE: 4).9
Types of skin malignancies
See Photo Rounds, page 219, for images of many types of skin cancer.
Basal cell carcinoma
The patterns of basal cell carcinoma are nodular, superficial, micronodular, infiltrative, morpheaform, and mixed.10 They may be pigmented and are sometimes misdiagnosed as melanoma.11 However, most basal cell carcinomas are typical in appearance and easily diagnosed by visual and tactile inspection.
The most common nodular type is a smooth, skin-colored, indurated, dome-shaped papule with a rolled edge. Other attributes include a pearly appearance, overlying telangiectatic vessels, and a history of bleeding with minor trauma.7,11
Superficial basal cell carcinoma is similar to dermatitis but more often has distinct borders and a bright pink appearance.11 If in doubt about the diagnosis, obtain a tissue sample for pathology.
Squamous cell carcinoma
Squamous cell carcinoma most often is a small, firm, hyperkaratotic nodule sitting atop an inflamed base. It may also be skin-colored and smooth. The history can include itching, pain, and nonhealing after minor trauma.7,11,12 As with basal cell carcinoma, diagnosis is made by tissue pathology.
Malignant melanoma
Malignant melanoma usually appears as a changing or unusual mole with haphazard color variegation, including combinations of brown, black, blue, gray, white, and (rarely) pink. Most melanomas are larger than 5 mm in diameter at time of diagnosis.13
There are 4 main types of malignant melanoma:
- Superficial spreading melanoma accounts for 50% of cases and occurs more frequently in younger adults.
- Nodular melanoma also occurs in younger adults, representing 20% to 25% of cases.
- Lentigo maligna melanoma occurs in older adults and accounts for only 15% of cases.
- Acral or acral-lentiginous melanomas are the least common form (10% of cases). They appear on the palms, soles, and around the first toenail.14
Risk factors for skin malignancies
Factors conferring the highest relative risk for malignant melanoma include:13
- atypical nevus syndrome with a personal and family history of melanoma
- history of a changing mole
- atypical nevus syndrome with just a family history of melanoma
- age greater than or equal to 15 years
- history of dysplastic moles.
Table 1 provides a list of risk factors that should prompt an annual skin survey (LOE: 5).
For nonmelanoma skin cancers, the strongest risk factors ( Table 2) include Caucasian race; age 55 to 75 years; and male sex.2 There is good evidence that a history of nonmelanoma skin cancer confers a 10-fold risk for recurrence (LOE: 2a).15 A distinct risk factor for squamous cell carcinoma is immunosuppression.2 Table 2 also provides a complete list of risk factors for nonmelanoma skin cancer.
Precursor lesions for nonmelanoma skin cancers include Bowen’s disease and erythroplasia of Queyrat (forms of squamous cell carcinoma in situ that will progress if left untreated). Actinic keratoses are common precursor lesions, but their overall annual rate of malignant transformation is only 1 in 400. In the case of SCC, up to 60% of cancers develop from an existing actinic keratosis.2
TABLE 1
Risk factors for malignant melanoma 13
| Risk factors that should prompt an annual skin survey | RR (LOE)* |
|---|---|
| Atypical nevus syndrome with personal and family history of melanoma | 500 (1b) |
| Changing mole | >400 (4) |
| Atypical nevus syndrome with family history of melanoma | 148 (1b)† |
| Age ≥ 15 | 88 (2c) |
| Dysplastic moles | 7–70 (3b) |
| History of melanoma before age 40 | 23 (2b) |
| Large congenital nevus (≥15 cm) | 17 (2b) |
| Caucasian race | 12 (2b) |
| Lentigo maligna | 10 (2c) |
| Atypical nevi | 7–27 (3b) |
| Regular use of tanning bed before age 30 | 7.7‡ (3b) |
| Multiple nevi | 5–12 (3b) |
| Personal history of melanoma | 5–9 (2b) |
| Immunosupression | 4–8 (2b) |
| Family history (first degree) of melanoma | 3–8 (3b) |
| Nonmelanoma skin cancer | 3–5 (3b) |
| Sun sensitivity or tendency to burn | 2–3 (3b) |
| *See page 239 for a description of levels of evidence | |
| †(95% CI, 40–379) | |
| ‡(95% CI, 1–63.6) | |
| RR, relative risk (compared with person without risk factors); | |
| LOE, level of evidence; | |
| CI, confidence interval | |
TABLE 2
Risk factors for nonmelanoma skin cancer
| Significant risk factors | RR | LOE* |
|---|---|---|
| Caucasian race | 70 | 2c |
| Immunosuppression | 5–20 | 2c |
| Previous nonmelanoma skin cancer | 10 | 2a |
| Age 55–75 | 4–8 | 2c |
| Male sex | 2 | 2c |
| Genetic risk factors associated with nonmelonoma skin cancer 3 | ||
| ||
| Chemical exposure risk factors associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| Environmental factors and medical conditions associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| *See page 239 for a description of levels of evidence | ||
| RR, relative risk (compared with person without risk factors); LOE, level of evidence | ||
Clinical prediction rules for skin malignancies
Malignant melanoma
ABCDE criteria. A useful clinical prediction rule for malignant melanoma is the American Cancer Society’s “ABCDE criteria” (Table 3). This rule was validated in 4 dermatology clinics, studying a total of 1118 lesions, although the studies were not homogenous (strength of recommendation [SOR]: A).16-19 Results of the study are summarized in Table 4. The test is normally considered positive if one or more of the criteria are met; however, as more criteria are met, specificity increases while sensitivity decreases.17-19
For lesions lacking any of the ABCDE criteria, 99.8% are something other than melanoma (using a prevalence of 1% found in the US population) (SOR: A). Use caution, however, as this rule will miss amelanotic melanomas, as well as smaller melanomas that are changing in size or have other features suggestive of malignant melanoma.
Conversely, if one of the criteria is met, there is nearly a 1.5% (positive predictive value) probability it is melanoma. Excisional biopsy of the lesion is indicated if good clinical judgment is used and it cannot be identified with certainty as a typical benign lesion (SOR: A). This test thus guides clinicians when making a decision to biopsy, as well as in choosing a biopsy technique.
The ABCDE criteria establish a risk of malignancy if the lesion is 6 mm in diameter or greater. Some evidence, however, suggests that this value should not be used as an absolute cutoff for diagnosing malignant melanoma. A large retrospective study performed in Australia found that 31% of biopsy-confirmed melanomas were less than 6 mm in diameter (LOE: 2b).20
Revised 7-point checklist. Another potentially useful diagnostic test is the revised 7-point checklist developed in the United Kingdom ( Table 5). This test was found to have a high sensitivity, but low specificity ( Table 4) . Therefore, it has a low false-negative rate, and is useful for ruling out the diagnosis of melanoma when negative. However, the test yields a significant number of false positive results, leading to possibly unnecessary biopsies and increased patient anxiety (SOR: B).16,21,22
Note: the described sensitivities and specificities for both tests apply only to malignant melanomas, and their accuracy decreases when including basal cell and squamous cell carcinomas. Also, the 2 tests were scored differently in some of the validation studies, making attempts to generalize problematic.
Studies of physicians’ global assessments to detect melanomas ( Table 4) vary widely for sensitivity (50% to 97%) but are consistent for specificity (96% to 99%) (SOR: B).23-28 Additionally, some studies have shown higher percentages of correct diagnosis of malignant melanoma among dermatologists compared with nondermatologists, but all these studies (except for a small subset of patients in one) used lesion images rather than patient examinations (SOR: B).23,29-33
More importantly, when the choice of correct treatment was evaluated, no statistically significant difference was found between the two groups. Further prospective cohort trials using patient examinations are needed to evaluate dermatologist performance versus nondermatologist performance.
TABLE 3
American Cancer Society's ABCDE criteria
| The test is considered positive if a lesion exhibits 1 or more of the 5 criteria |
| Assymetry—one half of the lesion not identical to the other |
| Border irregularity—lesion has an uneven or ragged border |
| Color variegation—lesion has more than one color (ie, black, blue, pink, red, or white) |
| Diameter—lesion has a diameter greater than 6 mm |
| Elevation or Enlargement—elevation of lesion above skin surface or enlargement by patient report |
 |
TABLE 4
Clinical prediction tests for skin malignancies
| Diagnostic test | Study quality (SOR)* | Sensitivity % (average) | Specificity % (average) | LR+ (1% pretest) | LR– | PV+ | PV– |
|---|---|---|---|---|---|---|---|
| ABCDE criteria (1 criterion positive)16-19 | A | 92–97 (93) | 13–63 (37) | 1.5 | 0.2 | 1.5% | 99.8% |
| Revised 7-point checklist16,21,22 | B | 79–100 (90) | 30–37 (34) | 1.4 | 0.3 | 1.4% | 99.7% |
| Physician global assessments23-28 | B | 50–97 (74) | 96–99 (98) | 37 | 0.3 | 27.2% | 99.7% |
| *See page 239 for a description of strength of recommendation | |||||||
| Note: These calculations are based on simple averages. Statistical homogeneity could not be fully evaluated due to study data limitations. | |||||||
| SOR, strength of recommendation; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, positive predictive value; PV–, negative predictive value | |||||||
TABLE 5
Revised 7-point checklist for assessing risk of melanoma
Suspect melanoma if there are 1 or more major signs:
|
3 or 4 minor signs without a major sign can also indicate a need to biopsy suspicious moles:
|
No validated tool for diagnosis of nonmelanoma skin cancers
A useful diagnostic tool has not yet been validated for nonmelanoma skin cancers. Over 60% of non-melanoma skin cancers occur on the face and neck, and these areas bear careful inspection. Lesions behind the ear, at the medial canthus, and within the nasolabial folds are most easily missed.
How to proceed in assessing lesions
When evaluating skin lesions, remember the gold standard for diagnosis of skin malignancies is a tissue biopsy. If you or your patient has any doubt about the diagnosis, a biopsy should be performed.
To review: Good evidence supports the use the ABCDE criteria or the revised 7-point checklist in determining whether lesions are likely to be malignant melanomas. No similar diagnostic rules exist for basal cell and squamous cell carcinomas. The decision to biopsy these lesions must be based on global assessment and typical characteristics.
Based on this information, we developed an algorithm for evaluating patients at risk for skin malignancies ( Figure) . The first step is to apply the ABCDE criteria and the revised 7-point checklist to identify or rule out possible malignant melanomas. An excisional biopsy should be performed if either test is positive (and the lesion is not clinically benign), or if you or your patient has any doubt.
If neither of these diagnostic tests yields a positive result, the lesion should be classified as typically benign or as having characteristics suggestive of a squamous cell or basal cell carcinoma.
Lesions that have characteristics of squamous cell or basal cell cancer should be biopsied, and benign lesions can be observed and the patient reassured.
FIGURE
Approach to the patient with a skin lesion
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript. We also gratefully acknowledge Dr. Richard P. Usatine for preparing the accompanying Photo Rounds.
- Expect to encounter 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma ever y year.
- There is good evidence for using the American Cancer Society’s ABCDE criteria as a clinical diagnostic test to rule out malignant melanoma (A).
- The revised 7-point checklist has high sensitivity and is therefore useful for ruling out a diagnosis of malignant melanoma. However, its low specificity yields many false-positive results (B).
- The gold standard for diagnosis of skin malignancies is a tissue biopsy. If any doubt exists about the diagnosis, a biopsy should be performed (A).
The American Cancer Society’s ABCDE criteria and the revised 7-point checklist are the most reliable means of detecting or ruling out malignant melanoma. Each has its strengths and weaknesses, a knowledge of which will increase the accuracy of assessment and minimize chances of misdiagnosis.
In addition to these 2 clinical prediction rules, we examine the evidence on physician’s global assessment of nonmelanoma skin cancers and review the risk factors for the major types of skin cancer. As a result of a comprehensive evidence-based review on the incidence, risk factors, and diagnosis of skin malignancies, we present an algorithm for evaluating skin lesions.
Impact of skin cancer
The incidence of malignant melanoma has increased from 1 in 1500 in 1930 to 1 in 75 for the year 2000.1 Although it is the rarest skin cancer (1% of skin malignancies), it is also the deadliest, accounting for 60% of skin cancer deaths.2
Nonmelanoma skin cancers, which include squamous cell cancers and basal cell cancers, account for one third of all cancers in the United States. Approximately 1 million cases were diagnosed in 1999.3 Deaths from nonmelanoma skin cancers are in steady decline, and the overall 5-year survival rate is high (over 95%).4 Recurrent nonmelanoma skin cancer, however, carries a very poor prognosis, with only a 50% cure rate.5
Treatment of nonmelanoma skin cancer costs over $500 million yearly in the US.4
Primary care physicians help improve prognosis
More persons visit primary care physicians (38.2%) than dermatologists (29.9%) for evaluation of suspicious skin lesions.6 Such lesions are usually benign, but a malignancy must be excluded. A primary care physician can expect to diagnose 6 to 7 cases of basal cell cancer, 1 to 2 cases of squamous cell cancer, and approximately 1 case of melanoma every year, according to population-based studies.4
Primary care practitioners contribute to a more favorable prognosis. For each additional family physician per 10,000 population, the chances of diagnosing malignant melanoma earlier increase significantly (odds ratio= 1.21, 95% confidence interval, 1.09–1.33, P<.001).7
Primary care physicians who diagnose non-melanoma skin cancers can select therapies that offer maximum efficacy and cost-effectiveness.
Differential diagnosis
According to a study of 1215 biopsies conducted in a primary care population, over 80% of biopsied lesions were benign and included nevi, seborrheic keratoses, cysts, dermatofibromas, fibrous histiocytomas, and polyps or skin tags. Pre-malignant lesions (including actinic keratoses and lentigo maligna) represented 7% of the total. Thirteen percent were malignancies: basal cell carcinomas (73%), followed by squamous cell carcinomas (14%), and malignant melanomas (12%). One metastatic adenomacarcinoma was included in the series (1%) (level of evidence [LOE]: 4).8
The differential diagnosis for basal cell carcinoma includes superficial basal cell carcinoma, pigmented basal cell carcinoma, infiltrating basal cell carcinoma, tricoepithelioma, keloid, molluscum contagiosum, and dermatofibromas.
For squamous cell cancer, the differential includes squamous cell carcinoma, keratoacanthoma, eczema and atopic dermatitis, contact dermatitis, psoriasis, and seborrheic dermatitis.
The differential diagnosis for malignant melanoma includes seborrheic keratosis, traumatized or irritated nevus, pigmented basal cell carcinoma, lentigo, blue nevus, angiokeratoma, traumatic hematoma, venous lake, hemangioma, dermatofibroma, and pigmented actinic keratosis.
Using the history and physical examination
Nearly 70% of melanomas are discovered by patients or their family (LOE: 4).9 Patients may express concern about changed size or appearance of a lesion; associated pain, pruritis, ulceration, or bleeding; location in a cosmetically sensitive area; or worry voiced by a family member. Additionally, a patient may have a family or personal history of skin malignancy, history of skin biopsy, or predisposition to sunburns.
Nurses and physicians identify lesions before a patient does approximately one quarter of the time while examining a patient for an unrelated condition or as part of a comprehensive work-up (LOE: 4).9
Types of skin malignancies
See Photo Rounds, page 219, for images of many types of skin cancer.
Basal cell carcinoma
The patterns of basal cell carcinoma are nodular, superficial, micronodular, infiltrative, morpheaform, and mixed.10 They may be pigmented and are sometimes misdiagnosed as melanoma.11 However, most basal cell carcinomas are typical in appearance and easily diagnosed by visual and tactile inspection.
The most common nodular type is a smooth, skin-colored, indurated, dome-shaped papule with a rolled edge. Other attributes include a pearly appearance, overlying telangiectatic vessels, and a history of bleeding with minor trauma.7,11
Superficial basal cell carcinoma is similar to dermatitis but more often has distinct borders and a bright pink appearance.11 If in doubt about the diagnosis, obtain a tissue sample for pathology.
Squamous cell carcinoma
Squamous cell carcinoma most often is a small, firm, hyperkaratotic nodule sitting atop an inflamed base. It may also be skin-colored and smooth. The history can include itching, pain, and nonhealing after minor trauma.7,11,12 As with basal cell carcinoma, diagnosis is made by tissue pathology.
Malignant melanoma
Malignant melanoma usually appears as a changing or unusual mole with haphazard color variegation, including combinations of brown, black, blue, gray, white, and (rarely) pink. Most melanomas are larger than 5 mm in diameter at time of diagnosis.13
There are 4 main types of malignant melanoma:
- Superficial spreading melanoma accounts for 50% of cases and occurs more frequently in younger adults.
- Nodular melanoma also occurs in younger adults, representing 20% to 25% of cases.
- Lentigo maligna melanoma occurs in older adults and accounts for only 15% of cases.
- Acral or acral-lentiginous melanomas are the least common form (10% of cases). They appear on the palms, soles, and around the first toenail.14
Risk factors for skin malignancies
Factors conferring the highest relative risk for malignant melanoma include:13
- atypical nevus syndrome with a personal and family history of melanoma
- history of a changing mole
- atypical nevus syndrome with just a family history of melanoma
- age greater than or equal to 15 years
- history of dysplastic moles.
Table 1 provides a list of risk factors that should prompt an annual skin survey (LOE: 5).
For nonmelanoma skin cancers, the strongest risk factors ( Table 2) include Caucasian race; age 55 to 75 years; and male sex.2 There is good evidence that a history of nonmelanoma skin cancer confers a 10-fold risk for recurrence (LOE: 2a).15 A distinct risk factor for squamous cell carcinoma is immunosuppression.2 Table 2 also provides a complete list of risk factors for nonmelanoma skin cancer.
Precursor lesions for nonmelanoma skin cancers include Bowen’s disease and erythroplasia of Queyrat (forms of squamous cell carcinoma in situ that will progress if left untreated). Actinic keratoses are common precursor lesions, but their overall annual rate of malignant transformation is only 1 in 400. In the case of SCC, up to 60% of cancers develop from an existing actinic keratosis.2
TABLE 1
Risk factors for malignant melanoma 13
| Risk factors that should prompt an annual skin survey | RR (LOE)* |
|---|---|
| Atypical nevus syndrome with personal and family history of melanoma | 500 (1b) |
| Changing mole | >400 (4) |
| Atypical nevus syndrome with family history of melanoma | 148 (1b)† |
| Age ≥ 15 | 88 (2c) |
| Dysplastic moles | 7–70 (3b) |
| History of melanoma before age 40 | 23 (2b) |
| Large congenital nevus (≥15 cm) | 17 (2b) |
| Caucasian race | 12 (2b) |
| Lentigo maligna | 10 (2c) |
| Atypical nevi | 7–27 (3b) |
| Regular use of tanning bed before age 30 | 7.7‡ (3b) |
| Multiple nevi | 5–12 (3b) |
| Personal history of melanoma | 5–9 (2b) |
| Immunosupression | 4–8 (2b) |
| Family history (first degree) of melanoma | 3–8 (3b) |
| Nonmelanoma skin cancer | 3–5 (3b) |
| Sun sensitivity or tendency to burn | 2–3 (3b) |
| *See page 239 for a description of levels of evidence | |
| †(95% CI, 40–379) | |
| ‡(95% CI, 1–63.6) | |
| RR, relative risk (compared with person without risk factors); | |
| LOE, level of evidence; | |
| CI, confidence interval | |
TABLE 2
Risk factors for nonmelanoma skin cancer
| Significant risk factors | RR | LOE* |
|---|---|---|
| Caucasian race | 70 | 2c |
| Immunosuppression | 5–20 | 2c |
| Previous nonmelanoma skin cancer | 10 | 2a |
| Age 55–75 | 4–8 | 2c |
| Male sex | 2 | 2c |
| Genetic risk factors associated with nonmelonoma skin cancer 3 | ||
| ||
| Chemical exposure risk factors associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| Environmental factors and medical conditions associated with nonmelonoma skin cancer (particularly squamous cell carcinoma) 3 | ||
| ||
| *See page 239 for a description of levels of evidence | ||
| RR, relative risk (compared with person without risk factors); LOE, level of evidence | ||
Clinical prediction rules for skin malignancies
Malignant melanoma
ABCDE criteria. A useful clinical prediction rule for malignant melanoma is the American Cancer Society’s “ABCDE criteria” (Table 3). This rule was validated in 4 dermatology clinics, studying a total of 1118 lesions, although the studies were not homogenous (strength of recommendation [SOR]: A).16-19 Results of the study are summarized in Table 4. The test is normally considered positive if one or more of the criteria are met; however, as more criteria are met, specificity increases while sensitivity decreases.17-19
For lesions lacking any of the ABCDE criteria, 99.8% are something other than melanoma (using a prevalence of 1% found in the US population) (SOR: A). Use caution, however, as this rule will miss amelanotic melanomas, as well as smaller melanomas that are changing in size or have other features suggestive of malignant melanoma.
Conversely, if one of the criteria is met, there is nearly a 1.5% (positive predictive value) probability it is melanoma. Excisional biopsy of the lesion is indicated if good clinical judgment is used and it cannot be identified with certainty as a typical benign lesion (SOR: A). This test thus guides clinicians when making a decision to biopsy, as well as in choosing a biopsy technique.
The ABCDE criteria establish a risk of malignancy if the lesion is 6 mm in diameter or greater. Some evidence, however, suggests that this value should not be used as an absolute cutoff for diagnosing malignant melanoma. A large retrospective study performed in Australia found that 31% of biopsy-confirmed melanomas were less than 6 mm in diameter (LOE: 2b).20
Revised 7-point checklist. Another potentially useful diagnostic test is the revised 7-point checklist developed in the United Kingdom ( Table 5). This test was found to have a high sensitivity, but low specificity ( Table 4) . Therefore, it has a low false-negative rate, and is useful for ruling out the diagnosis of melanoma when negative. However, the test yields a significant number of false positive results, leading to possibly unnecessary biopsies and increased patient anxiety (SOR: B).16,21,22
Note: the described sensitivities and specificities for both tests apply only to malignant melanomas, and their accuracy decreases when including basal cell and squamous cell carcinomas. Also, the 2 tests were scored differently in some of the validation studies, making attempts to generalize problematic.
Studies of physicians’ global assessments to detect melanomas ( Table 4) vary widely for sensitivity (50% to 97%) but are consistent for specificity (96% to 99%) (SOR: B).23-28 Additionally, some studies have shown higher percentages of correct diagnosis of malignant melanoma among dermatologists compared with nondermatologists, but all these studies (except for a small subset of patients in one) used lesion images rather than patient examinations (SOR: B).23,29-33
More importantly, when the choice of correct treatment was evaluated, no statistically significant difference was found between the two groups. Further prospective cohort trials using patient examinations are needed to evaluate dermatologist performance versus nondermatologist performance.
TABLE 3
American Cancer Society's ABCDE criteria
| The test is considered positive if a lesion exhibits 1 or more of the 5 criteria |
| Assymetry—one half of the lesion not identical to the other |
| Border irregularity—lesion has an uneven or ragged border |
| Color variegation—lesion has more than one color (ie, black, blue, pink, red, or white) |
| Diameter—lesion has a diameter greater than 6 mm |
| Elevation or Enlargement—elevation of lesion above skin surface or enlargement by patient report |
 |
TABLE 4
Clinical prediction tests for skin malignancies
| Diagnostic test | Study quality (SOR)* | Sensitivity % (average) | Specificity % (average) | LR+ (1% pretest) | LR– | PV+ | PV– |
|---|---|---|---|---|---|---|---|
| ABCDE criteria (1 criterion positive)16-19 | A | 92–97 (93) | 13–63 (37) | 1.5 | 0.2 | 1.5% | 99.8% |
| Revised 7-point checklist16,21,22 | B | 79–100 (90) | 30–37 (34) | 1.4 | 0.3 | 1.4% | 99.7% |
| Physician global assessments23-28 | B | 50–97 (74) | 96–99 (98) | 37 | 0.3 | 27.2% | 99.7% |
| *See page 239 for a description of strength of recommendation | |||||||
| Note: These calculations are based on simple averages. Statistical homogeneity could not be fully evaluated due to study data limitations. | |||||||
| SOR, strength of recommendation; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, positive predictive value; PV–, negative predictive value | |||||||
TABLE 5
Revised 7-point checklist for assessing risk of melanoma
Suspect melanoma if there are 1 or more major signs:
|
3 or 4 minor signs without a major sign can also indicate a need to biopsy suspicious moles:
|
No validated tool for diagnosis of nonmelanoma skin cancers
A useful diagnostic tool has not yet been validated for nonmelanoma skin cancers. Over 60% of non-melanoma skin cancers occur on the face and neck, and these areas bear careful inspection. Lesions behind the ear, at the medial canthus, and within the nasolabial folds are most easily missed.
How to proceed in assessing lesions
When evaluating skin lesions, remember the gold standard for diagnosis of skin malignancies is a tissue biopsy. If you or your patient has any doubt about the diagnosis, a biopsy should be performed.
To review: Good evidence supports the use the ABCDE criteria or the revised 7-point checklist in determining whether lesions are likely to be malignant melanomas. No similar diagnostic rules exist for basal cell and squamous cell carcinomas. The decision to biopsy these lesions must be based on global assessment and typical characteristics.
Based on this information, we developed an algorithm for evaluating patients at risk for skin malignancies ( Figure) . The first step is to apply the ABCDE criteria and the revised 7-point checklist to identify or rule out possible malignant melanomas. An excisional biopsy should be performed if either test is positive (and the lesion is not clinically benign), or if you or your patient has any doubt.
If neither of these diagnostic tests yields a positive result, the lesion should be classified as typically benign or as having characteristics suggestive of a squamous cell or basal cell carcinoma.
Lesions that have characteristics of squamous cell or basal cell cancer should be biopsied, and benign lesions can be observed and the patient reassured.
FIGURE
Approach to the patient with a skin lesion
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript. We also gratefully acknowledge Dr. Richard P. Usatine for preparing the accompanying Photo Rounds.
1. Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol 1996;34:839-847.
2. Skin Tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases Philadelphia, Pa: Lippincott-Raven, 1996;342.-
3. Landis SH, Murray T, Bolden S, Wingo PA. Cancer Statistics, 1999. CA Cancer J Clin 1999;49:8-31.
4. Gloster HM, , Jr. Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-96 Summary. Vital Health Stat 1999;13:1-122.
7. Roetzheim RG, Naazneen P, Van Durme DJ. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol 2000;43:211-218.
8. Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. JABFP 1996;9:397-404.
9. Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma: patterns from a population-based survey. J Am Acad Dermatol 1992;26:914-919.
10. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
11. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
12. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
13. Rhodes AR, Weinstock MA, , Jr, Fitzpatrick B, Mihm MC, Jr, Sober AJ. Risk factors for cutaneous melanoma: a practical method of recognizing predisposed individuals. JAMA 1987;258:3146-3154.
14. Austoker J. Melanoma: prevention and early diagnosis. BMJ 1994;308:1682-1686.
15. Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol 2000;136:1524-1530.
16. Healsmith MF, Bourke JF, Osborne JE, Graham-Brown RAC. An evaluation of the revised seven-point checklist for the early diagnosis of cutaneous melanoma. Br J Dermatol 1994;130:48-50.
17. McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions: a comparson of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesions. J Dermatol Surg Oncol 1992;18:22-26.
18. Benelli C, Roscetti E, Dal Pozzo V, Gasparini G, Cavicchini S. The dermoscopic versus the clinical diagnosis of melanoma. Eur J Dermatol 1999;9:470-476.
19. Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology 1998;197:11-17.
20. Shaw HM, McCarthy WH. Small-diameter malignant melanoma: a common diagnosis in New South Wales, Australia. J Am Acad Dermatol 1992;27:679-682.
21. Du Vivier AWP, Williams HC, Brett JV, Higgins EM. How do malignant melanomas present and does this correlate with the seven-point checklist? Clin Exp Dermatol 1991;16:344-347.
22. Higgins EM, Hall P, Todd P, Murthi R, Du Vivier AWP. The application of the seven-point check-list in the assessment of benign pigmented lesions. Clin Exp Dermatol 1992;17:313-315.
23. Whited JD, Grichnik JM. Does this patient have a more or a melanoma? JAMA 2002;279:696-701.
24. Curley RK, Cook MG, Fallowfield ME, Marsden RA. Accuracy in clinically evaluating pigmented lesions. Br Med J 1989;299:16-18.
25. DeCoste SD, Stern RS. Diagnosis and treatment of nevomelanocytic lesion of the skin: a community-based study. Arch Dermatol 1993;129:57-62.
26. Grin CM, Kopf AW, Welkovich B, Bart RS, Levenstein MJ. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol 1990;126:763-766.
27. Koh HK, Caruso A, Gage I, Geller AC, Prout MN, White H, et al. Evaluation of melanoma/skin cancer screening in Massachusetts: preliminary results. Cancer 1990;65:375-379.
28. McMullan FH, Hubener LF. Malignant melanoma: a statistical review of clinical and histological diagnoses. Arch Dermatol 1956;74:618-619.
29. Cassileth BR, Clark WHJ, Lusk EJ, Frederick BE, Thompson CJ, Walsh WP. How well do physicians recognize melanoma and other problem lesions? J Am Acad Dermatol 1986;14:555-560.
30. Gerbert G, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, et al. Primary care physicians as gatekeepers in managed care. Arch Dermatol 1996;132:1030-1038.
31. McGee R, Elwood M, Sneyd MJ, Williams S, Tilyard M. The recognition and management of melanoma and other skin lesions by general practitioners in New Zealand. N Z Med J 1994;107:287-290.
32. Paine SL, Cockburn J, Noy SM, Marks R. Early detection of skin cancer: knowledge, perceptions, and practices of general practitioners in Victoria. Med J Aust 1994;161:188-195.
33. Ramsay DL, Fox AB. The ability of primary care physicians to recognize the common dermatoses. Arch Dermatol 1981;117:620-622.
1. Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol 1996;34:839-847.
2. Skin Tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases Philadelphia, Pa: Lippincott-Raven, 1996;342.-
3. Landis SH, Murray T, Bolden S, Wingo PA. Cancer Statistics, 1999. CA Cancer J Clin 1999;49:8-31.
4. Gloster HM, , Jr. Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-96 Summary. Vital Health Stat 1999;13:1-122.
7. Roetzheim RG, Naazneen P, Van Durme DJ. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol 2000;43:211-218.
8. Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. JABFP 1996;9:397-404.
9. Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma: patterns from a population-based survey. J Am Acad Dermatol 1992;26:914-919.
10. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
11. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
12. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
13. Rhodes AR, Weinstock MA, , Jr, Fitzpatrick B, Mihm MC, Jr, Sober AJ. Risk factors for cutaneous melanoma: a practical method of recognizing predisposed individuals. JAMA 1987;258:3146-3154.
14. Austoker J. Melanoma: prevention and early diagnosis. BMJ 1994;308:1682-1686.
15. Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol 2000;136:1524-1530.
16. Healsmith MF, Bourke JF, Osborne JE, Graham-Brown RAC. An evaluation of the revised seven-point checklist for the early diagnosis of cutaneous melanoma. Br J Dermatol 1994;130:48-50.
17. McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions: a comparson of the Glasgow seven-point checklist and the American Cancer Society’s ABCDs of pigmented lesions. J Dermatol Surg Oncol 1992;18:22-26.
18. Benelli C, Roscetti E, Dal Pozzo V, Gasparini G, Cavicchini S. The dermoscopic versus the clinical diagnosis of melanoma. Eur J Dermatol 1999;9:470-476.
19. Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology 1998;197:11-17.
20. Shaw HM, McCarthy WH. Small-diameter malignant melanoma: a common diagnosis in New South Wales, Australia. J Am Acad Dermatol 1992;27:679-682.
21. Du Vivier AWP, Williams HC, Brett JV, Higgins EM. How do malignant melanomas present and does this correlate with the seven-point checklist? Clin Exp Dermatol 1991;16:344-347.
22. Higgins EM, Hall P, Todd P, Murthi R, Du Vivier AWP. The application of the seven-point check-list in the assessment of benign pigmented lesions. Clin Exp Dermatol 1992;17:313-315.
23. Whited JD, Grichnik JM. Does this patient have a more or a melanoma? JAMA 2002;279:696-701.
24. Curley RK, Cook MG, Fallowfield ME, Marsden RA. Accuracy in clinically evaluating pigmented lesions. Br Med J 1989;299:16-18.
25. DeCoste SD, Stern RS. Diagnosis and treatment of nevomelanocytic lesion of the skin: a community-based study. Arch Dermatol 1993;129:57-62.
26. Grin CM, Kopf AW, Welkovich B, Bart RS, Levenstein MJ. Accuracy in the clinical diagnosis of malignant melanoma. Arch Dermatol 1990;126:763-766.
27. Koh HK, Caruso A, Gage I, Geller AC, Prout MN, White H, et al. Evaluation of melanoma/skin cancer screening in Massachusetts: preliminary results. Cancer 1990;65:375-379.
28. McMullan FH, Hubener LF. Malignant melanoma: a statistical review of clinical and histological diagnoses. Arch Dermatol 1956;74:618-619.
29. Cassileth BR, Clark WHJ, Lusk EJ, Frederick BE, Thompson CJ, Walsh WP. How well do physicians recognize melanoma and other problem lesions? J Am Acad Dermatol 1986;14:555-560.
30. Gerbert G, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, et al. Primary care physicians as gatekeepers in managed care. Arch Dermatol 1996;132:1030-1038.
31. McGee R, Elwood M, Sneyd MJ, Williams S, Tilyard M. The recognition and management of melanoma and other skin lesions by general practitioners in New Zealand. N Z Med J 1994;107:287-290.
32. Paine SL, Cockburn J, Noy SM, Marks R. Early detection of skin cancer: knowledge, perceptions, and practices of general practitioners in Victoria. Med J Aust 1994;161:188-195.
33. Ramsay DL, Fox AB. The ability of primary care physicians to recognize the common dermatoses. Arch Dermatol 1981;117:620-622.