User login
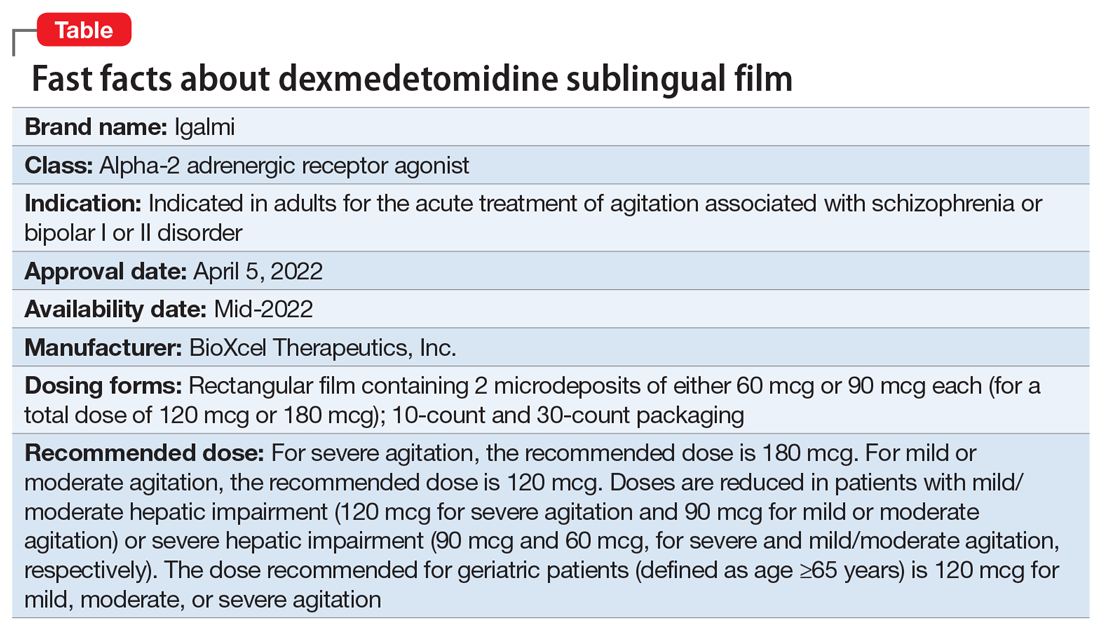
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
Approved by the FDA on April 5, 2022, dexmedetomidine sublingual film (Igalmi, manufactured and distributed by BioXcel Therapeutics, Inc., New Haven, CT USA) is indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder (Table).1,2 It is administered sublingually or buccally under the supervision of a health care provider. After administration, patients should have their vital signs and alertness assessed but there is no FDA Risk Evaluation and Mitigation Strategy (REMS) required for use. A limitation of use is that the safety and effectiveness of dexmedetomidine sublingual film has not been established beyond 24 hours from the first dose.2 There are no contraindications for use.2
Dexmedetomidine is a well-known efficacious alpha-2 adrenergic receptor agonist available since 1999 in an IV formulation indicated for sedation of initially intubated and mechanically ventilated patients in an ICU setting, and sedation of nonintubated patients prior to and/or during surgical and other procedures.3,4 The reformulation of dexmedetomidine as a sublingual film allows the broader use of this agent in psychiatric settings when managing agitation in patients with schizophrenia or bipolar disorder, and thus potentially avoiding the use of IM administration of antipsychotics and/or benzodiazepines. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.5
Dosing
Dexmedetomidine sublingual film is distributed commercially in the following strengths: 180 mcg and 120 mcg. It consists of a lightly mint-flavored, rectangular film containing 2 microdeposits of dexmedetomidine hydrochloride. Dosage strengths of 90 mcg and 60 mcg are available by cutting the 180 mcg or 120 mcg film in half
If agitation persists after the initial dose, up to 2 additional doses (90 mcg if the initial dose was 180 mcg, otherwise 60 mcg if the initial dose was 120, 90, or 60 mcg) may be given at least 2 hours apart. Assessment of vital signs, including orthostatic measurements, is required prior to the administration of any subsequent doses. Due to risk of hypotension, additional doses are not recommended in patients with systolic blood pressure <90 mm Hg, diastolic blood pressure <60 mm Hg, heart rate <60 beats per minute, or postural decrease in systolic blood pressure ≥20 mm Hg or in diastolic blood pressure ≥10 mm Hg.
Mechanism of action and pharmacodynamics
Dexmedetomidine is an alpha-2 adrenergic receptor agonist and the mechanism of action in the acute treatment of agitation is thought to be due to activation of presynaptic alpha-2 adrenergic receptors.2 Binding affinities (Ki values) are 4 to 6 nM at the alpha-2 adrenergic receptor subtypes.2
Dexmedetomidine exhibits concentration-dependent QT prolongation, with mean QTc increases from baseline from 6 msec (120 mcg single dose) to 11 msec (180 mcg plus 2 additional doses of 90 mcg 2 hours apart for a total of 3 doses).2 Placing the observation about QTc prolongation into clinical context, studies of IM administration of ziprasidone 20 mg and 30 mg and haloperidol 7.5 mg and 10 mg resulted in changes of the QTc interval of 4.6 msec and 6.0 msec, respectively, after 1 dose.6 After a second injection, these values were 12.8 msec and 14.7 msec, respectively.6
Clinical pharmacokinetics
The sublingual film formulation is absorbed orally, bypassing first-pass metabolism, and achieving higher dexmedetomidine bioavailability than ingested formulations.7 Exposure is dose-dependent, with dexmedetomidine being quantifiable in plasma after 5 to 20 minutes post dosing, and with a plasma half-life of 2 to 3 hours.2,8 Mean time for the film to dissolve in the mouth was approximately 6 to 8 minutes following sublingual administration, and 18 minutes following buccal administration.2 Absolute bioavailability was approximately 72% and 82% following sublingual and buccal administration, respectively.2 Mean maximal plasma concentrations of dexmedetomidine were reached approximately 2 hours after sublingual or buccal administration.2 Compared to drinking water at 2 hours post administration, early water intake (as early as 15 minutes post-dose) had minimal effects on the rate or extent of sublingual absorption but was not assessed after buccal administration.2 The average protein binding was 94% and was constant across the different plasma concentrations evaluated and similar in males and females, but significantly decreased in participants with hepatic impairment compared to healthy individuals.2 In contrast, the pharmacokinetic profile of dexmedetomidine is not significantly different in patients with creatinine clearance <30 mL/minute compared to those with normal renal function.2 Dexmedetomidine undergoes almost complete biotransformation to inactive metabolites via direct glucuronidation as well as cytochrome P450 (CYP) (primarily CYP2A6)–mediated metabolism.2 There is no evidence of any CYP–mediated drug interactions that are likely to be of clinical relevance.2
Continue to: Efficacy
Efficacy
The efficacy and tolerability of 120 mcg and 180 mcg doses of dexmedetomidine sublingual film was evaluated in 2 similarly designed, randomized, double-blind, placebo-controlled, Phase 3 trials in the treatment of acute agitation associated with schizophrenia, schizoaffective, or schizophreniform disorder9 and bipolar I or II disorder.10 These studies included a total of 758 adult patients age range 18 to 71 (mean age approximately 46.5), with about 59% male participants.2 In contrast to other agents approved by the FDA for treatment of agitation associated with bipolar disorder, dexmedetomidine sublingual film was assessed in patients regardless of polarity (manic, mixed features, or depressed).5 The primary efficacy measure for the dexmedetomidine sublingual film studies was the investigator-administered Positive and Negative Syndrome Scale-Excited Component (PANSS-EC), consisting of the following 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control.11 The items from the PANSS-EC are rated from 1 (not present) to 7 (extremely severe) and thus the total scores range from 5 to 35. For enrollment in the studies, patients had to be judged to be clinically agitated with a total PANSS-EC score ≥14, with at least 1 individual item score ≥4.2
After study medication administration, the PANSS-EC was assessed from 10 minutes through 24 hours, with the primary endpoint being at 2 hours post-dose. Patients with schizophrenia or bipolar disorder who were treated with dexmedetomidine sublingual film 120 mcg or 180 mcg had superior symptomatic improvements from baseline to 2 hours post-dose compared to placebo, with treatment effects beginning as early as 20 to 30 minutes post-dose (for patients with schizophrenia, dexmedetomidine was statistically significantly superior to placebo beginning at 20 minutes following dosing with the 180 mcg dose and 30 minutes after the 120 mcg dose; for patients with bipolar disorder, differences from placebo were statistically significant beginning at 20 minutes after treatment with both the 120 mcg and 180 mcg doses).2 Evaluation of effect size for dexmedetomidine vs placebo for PANSS-EC response at 2 hours (defined as ≥40% improvement from baseline) resulted in a number needed to treat (NNT) of 3 when combining both studies and both doses,12 comparing favorably with the NNT values observed for IM formulations of aripiprazole, haloperidol, lorazepam, olanzapine, and ziprasidone,13 and inhaled loxapine.14
Overall tolerability and safety
The highlights of the prescribing information contain warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence.2 Advice is provided to ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation, a concern commonly raised when assessing potential treatments for agitation.15 Dexmedetomidine sublingual film should be avoided in patients with risk factors for prolonged QT interval, a precaution that was evident for the use of ziprasidone16 and where an effect is also noted with haloperidol.6 As per the prescribing information, the most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) are somnolence, oral paresthesia or oral hypoesthesia, dizziness, dry mouth, hypotension, and orthostatic hypotension. Rates of adverse reactions of somnolence (including fatigue and sluggishness) with dexmedetomidine 120 mcg or 180 mcg are almost the same (22% and 23%, respectively), and higher than the 6% observed with placebo.2 Other adverse reactions are substantially lower in frequency. These include oral paresthesia or oral hypoesthesia (6%, 7%, and 1%, for dexmedetomidine 120 mcg, 180 mcg, or placebo, respectively), dizziness (4%, 6%, 1%), hypotension (5%, 5%, 0%), orthostatic hypotension (3%, 5%, <1%), dry mouth (7%, 4%, 1%), nausea (2%, 3%, 2%), bradycardia (2%, 2%, 0%), and abdominal discomfort (0%, 2%, 1%).2
Regarding dose-dependent changes in blood pressure during the studies, 16%, 18%, and 9% of patients treated with 120 mcg, 180 mcg, and placebo, respectively, experienced orthostatic hypotension at 2 hours post dose. However, at 24 hours, none of the patients in the 180-mcg group experienced a systolic blood pressure ≤90 mm Hg with a decrease ≥20 mm Hg, compared with one patient (<1%) in the 120-mcg group and none in the placebo group.2
The prescribing information advises that concomitant use of dexmedetomidine sublingual film with anesthetics, sedatives, hypnotics, or opioids is likely to lead to enhanced CNS depressant effects, and that the prescriber should consider a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic, or opioid.2
Summary
Dexmedetomidine sublingual film is an oral medication indicated in adults for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder. The recommended dose depends on severity of agitation, age, and the presence of hepatic impairment. A dose of 180 mcg is recommended for severe agitation and a dose of 120 mcg is recommended for mild or moderate agitation, with doses adjusted lower in the presence of hepatic impairment. There are no contraindications but there are warnings and precautions regarding hypotension/orthostatic hypotension/bradycardia, QT interval prolongation, and somnolence. Clinicians should monitor vital signs and alertness after administration to prevent falls and syncope; however, there is no FDA REMS required for use. The clinical trial evidence supporting the use of dexmedetomidine is robust, with evidence of a treatment effect as early as 20 minutes after administration. Noninvasive formulations, although requiring cooperation from patients, have the potential to improve overall patient experience, thereby improving future cooperation between patients and health care professionals.
Bottom Line
Dexmedetomidine sublingual film provides an opportunity to rethink the approach to the management of agitation and avoid the potentially unnecessary use of IM injections. Dexmedetomidine sublingual film acts rapidly and is simple to use.
Related Resources
- Dexmedetomidine sublingual film (Iglami) prescribing information. https://www.igalmihcp.com/igalmi-pi.pdf
Drug Brand Names
Aripiprazole • Abilify
Dexmedetomidine • Igalmi, Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487
1. US Food and Drug Administration. NDA 215390 Approval Letter. Accessed April 5, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215390Orig1s000ltr.pdf
2. Igalmi [package insert]. BioXcel Therapeutics, Inc; 2022.
3. Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893-913. doi:10.1007/s40262-017-0507-7
4. Precedex [package insert]. Hospira, Inc; 2021.
5. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172. doi:10.5811/westjem.2015.12.28763
6. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491. doi:10.1016/j.clinthera.2010.03.003
7. Yocca F, DeVivo M, Seth S, et al. Dexmedetomidine—highly favorable pharmacokinetic and pharmacological features for a CNS therapeutic drug. Poster presented at: 58th Annual Meeting of the American College of Neuropsychopharmacology; December 8-11, 2019; Orlando, FL.
8. Adedoyin A, Preskorn S, Lathia CD. Pharmacokinetics of dexmedetomidine after a single sublingual dose of BXCL501 in patients with agitation associated with schizophrenia. Poster presented at: 23rd Annual Conference of the International Society for Bipolar Disorders; May 13-15, 2021. Virtual. Session 17.
9. Citrome LL, Lauriello J, Risinger R, et al. A novel rapidly effective treatment of agitation for schizophrenia with the oral dissolving film BXCL501. Poster presented at: American Psychiatric Association Annual Meeting; May 1-3, 2021. Virtual. Accessed November 11, 2021. https://www.psychiatry.org/File%20Library/Psychiatrists/Meetings/Annual-Meeting/2021/2021-APA-Annual-Meeting-Poster-Proceedings.pdf
10. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736. doi:10.1001/jama.2022.0799
11. Montoya A, Valladares A, Lizán L, et al. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes. 2011;9:18. doi:10.1186/1477-7525-9-18
12. Citrome L, Palko L, Hokett S, et al. Number needed to treat and number needed to harm from two phase 3 studies of BXCL501 for treating acute agitation in patients with schizophrenia and bipolar disorder. Poster presented at: Academy of Managed Care Pharmacy Nexus 2021; October 18-21, 2021; Denver, CO.
13. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885. doi:10.4088/jcp.v68n1207
14. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325. doi:10.1111/j.1742-1241.2011.02890.x
15. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry project Beta psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
16. Zimbroff DL, Allen MH, Battaglia J, et al. Best clinical practice with ziprasidone IM: update after 2 years of experience. CNS Spectr. 2005;10(9):1-15. doi:10.1017/s1092852900025487