User login
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.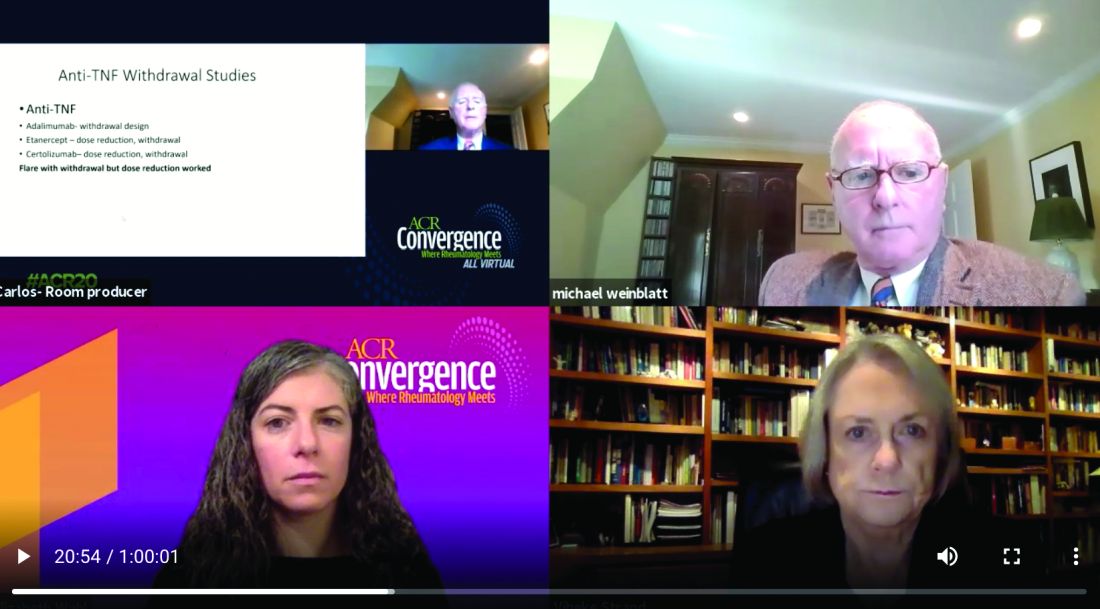
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
FROM ACR 2020