User login
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
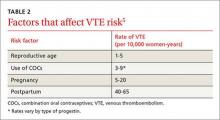
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
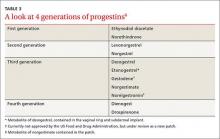
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; baturp@ccf.org.
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; baturp@ccf.org.
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; baturp@ccf.org.
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.