User login
The diagnosis
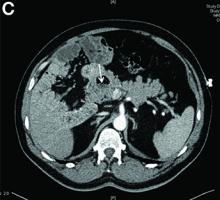
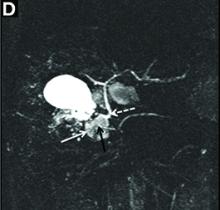
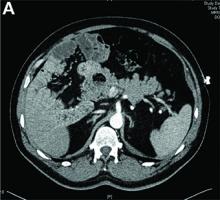
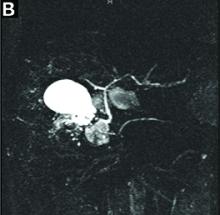
Answer to “What’s your diagnosis?” on page 2: Lemmel’s syndrome
References
1. Egawa, N., Anjiki, H., Takuma, K., et al. Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg. 2010;27:105-9.
2. Lobo, D.N., Balfour, T.W., Iftikhar, S.Y. Periampullary diverticula: consequences of failed ERCP. Ann Royal Coll Surg. 1998;80:326-31.
3. Lemmel, G. Die klinische Bedeutung der Duodenaldivertikel. Archiv fur Verdauungskrankheiten. 1934;56:59-70.
The diagnosis
Answer to “What’s your diagnosis?” on page 2: Lemmel’s syndrome
References
1. Egawa, N., Anjiki, H., Takuma, K., et al. Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg. 2010;27:105-9.
2. Lobo, D.N., Balfour, T.W., Iftikhar, S.Y. Periampullary diverticula: consequences of failed ERCP. Ann Royal Coll Surg. 1998;80:326-31.
3. Lemmel, G. Die klinische Bedeutung der Duodenaldivertikel. Archiv fur Verdauungskrankheiten. 1934;56:59-70.
The diagnosis
Answer to “What’s your diagnosis?” on page 2: Lemmel’s syndrome
References
1. Egawa, N., Anjiki, H., Takuma, K., et al. Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg. 2010;27:105-9.
2. Lobo, D.N., Balfour, T.W., Iftikhar, S.Y. Periampullary diverticula: consequences of failed ERCP. Ann Royal Coll Surg. 1998;80:326-31.
3. Lemmel, G. Die klinische Bedeutung der Duodenaldivertikel. Archiv fur Verdauungskrankheiten. 1934;56:59-70.
By Crispin Musumba, MBChB, PhD, Edward Britton, MBBS, MRCP, and Howard Smart, MBBS, DM. Published previously in Gastroenterology (2013;144:274, 468-469).