User login
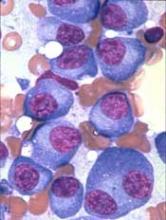
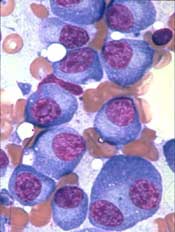
P-BCMA-101, an autologous chimeric antigen receptor (CAR) T-cell therapy being developed to treat patients with relapsed/refractory multiple myeloma (MM), has received regenerative medicine advanced therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA).
RMAT designation is intended to expedite the development and review of regenerative medicines that are intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition.
For a therapy to receive RMAT designation, preliminary evidence must indicate the therapy has the potential to address unmet medical needs for the disease or condition.
RMAT designation provides all the benefits of fast track and breakthrough therapy designations, including early interactions with the FDA.
About P-BCMA-101
To create P-BCMA-101, patients’ T cells are modified using a non-viral DNA modification system known as piggyBac™. The modified T cells target cells expressing B-cell maturation antigen (BCMA), which is expressed on essentially all MM cells.
Early results from the phase 1 trial (NCT03288493) of P-BCMA-101 were recently reported at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
The presentation included data on 11 patients with heavily pretreated MM. Patients were a median age of 60, and 73% were high risk. They had a median of six prior therapies.
Patients received conditioning with fludarabine and cyclophosphamide for 3 days prior to receiving P-BCMA-101. They then received one of three doses of CAR T cells—51×106 (n=3), 152×106 (n=7), or 430×106 (n=1).
The investigators observed no dose-limiting toxicities. Adverse events included neutropenia in eight patients and thrombocytopenia in five.
One patient may have had cytokine release syndrome, but the condition resolved without drug intervention. And investigators observed no neurotoxicity.
Seven of ten patients evaluable for response by International Myeloma Working Group criteria achieved at least a partial response, including very good partial responses and stringent complete response.
The eleventh patient has oligosecretory disease and was only evaluable by PET, which indicated a near complete response.
Additional results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1012).
The study is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
P-BCMA-101, an autologous chimeric antigen receptor (CAR) T-cell therapy being developed to treat patients with relapsed/refractory multiple myeloma (MM), has received regenerative medicine advanced therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA).
RMAT designation is intended to expedite the development and review of regenerative medicines that are intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition.
For a therapy to receive RMAT designation, preliminary evidence must indicate the therapy has the potential to address unmet medical needs for the disease or condition.
RMAT designation provides all the benefits of fast track and breakthrough therapy designations, including early interactions with the FDA.
About P-BCMA-101
To create P-BCMA-101, patients’ T cells are modified using a non-viral DNA modification system known as piggyBac™. The modified T cells target cells expressing B-cell maturation antigen (BCMA), which is expressed on essentially all MM cells.
Early results from the phase 1 trial (NCT03288493) of P-BCMA-101 were recently reported at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
The presentation included data on 11 patients with heavily pretreated MM. Patients were a median age of 60, and 73% were high risk. They had a median of six prior therapies.
Patients received conditioning with fludarabine and cyclophosphamide for 3 days prior to receiving P-BCMA-101. They then received one of three doses of CAR T cells—51×106 (n=3), 152×106 (n=7), or 430×106 (n=1).
The investigators observed no dose-limiting toxicities. Adverse events included neutropenia in eight patients and thrombocytopenia in five.
One patient may have had cytokine release syndrome, but the condition resolved without drug intervention. And investigators observed no neurotoxicity.
Seven of ten patients evaluable for response by International Myeloma Working Group criteria achieved at least a partial response, including very good partial responses and stringent complete response.
The eleventh patient has oligosecretory disease and was only evaluable by PET, which indicated a near complete response.
Additional results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1012).
The study is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
P-BCMA-101, an autologous chimeric antigen receptor (CAR) T-cell therapy being developed to treat patients with relapsed/refractory multiple myeloma (MM), has received regenerative medicine advanced therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA).
RMAT designation is intended to expedite the development and review of regenerative medicines that are intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition.
For a therapy to receive RMAT designation, preliminary evidence must indicate the therapy has the potential to address unmet medical needs for the disease or condition.
RMAT designation provides all the benefits of fast track and breakthrough therapy designations, including early interactions with the FDA.
About P-BCMA-101
To create P-BCMA-101, patients’ T cells are modified using a non-viral DNA modification system known as piggyBac™. The modified T cells target cells expressing B-cell maturation antigen (BCMA), which is expressed on essentially all MM cells.
Early results from the phase 1 trial (NCT03288493) of P-BCMA-101 were recently reported at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
The presentation included data on 11 patients with heavily pretreated MM. Patients were a median age of 60, and 73% were high risk. They had a median of six prior therapies.
Patients received conditioning with fludarabine and cyclophosphamide for 3 days prior to receiving P-BCMA-101. They then received one of three doses of CAR T cells—51×106 (n=3), 152×106 (n=7), or 430×106 (n=1).
The investigators observed no dose-limiting toxicities. Adverse events included neutropenia in eight patients and thrombocytopenia in five.
One patient may have had cytokine release syndrome, but the condition resolved without drug intervention. And investigators observed no neurotoxicity.
Seven of ten patients evaluable for response by International Myeloma Working Group criteria achieved at least a partial response, including very good partial responses and stringent complete response.
The eleventh patient has oligosecretory disease and was only evaluable by PET, which indicated a near complete response.
Additional results from this trial are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 1012).
The study is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.