User login
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
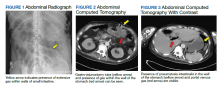
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011