User login
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
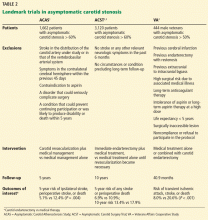
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
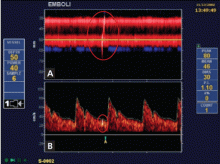
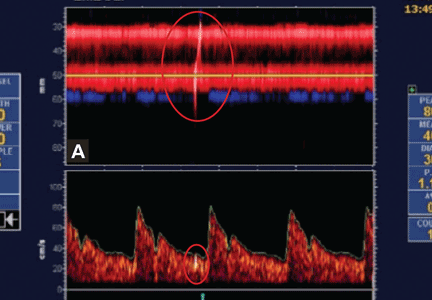
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
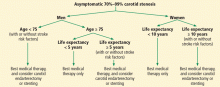
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
KEY POINTS
- Current guidelines are based on outdated data that may not represent the best evidence regarding the management of asymptomatic carotid disease.
- Stroke is a devastating outcome of carotid disease, and most patients and physicians are wary of deferring revascularization until a stroke occurs.
- Given the inherent risk associated with revascularization (endarterectomy or stenting) and the paucity of data, the approach should be personalized on the basis of life expectancy, sex, risk factors for stroke, and clinical acumen.
- Future research should focus on noninvasive tools to determine which patients are at high risk of stroke and may benefit from revascularization.