User login
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 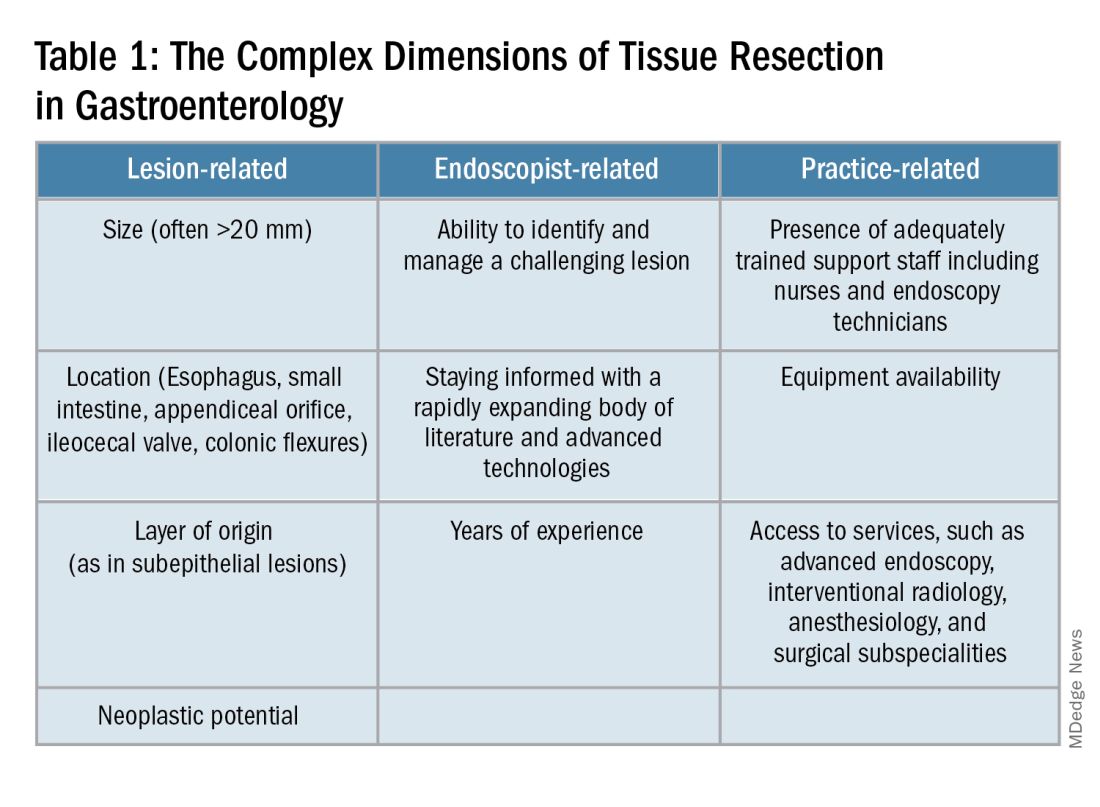
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1). 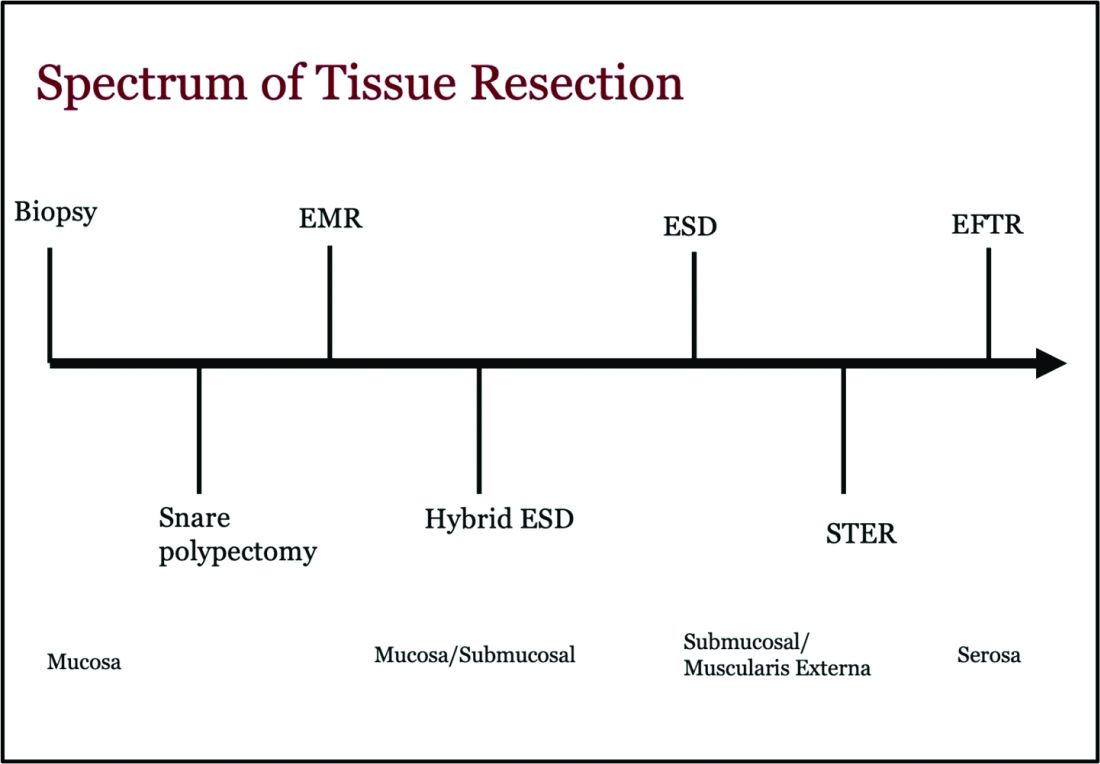
ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1). 
ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1). 
ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.


