User login
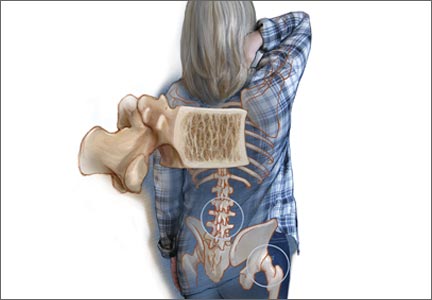
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.