User login
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.
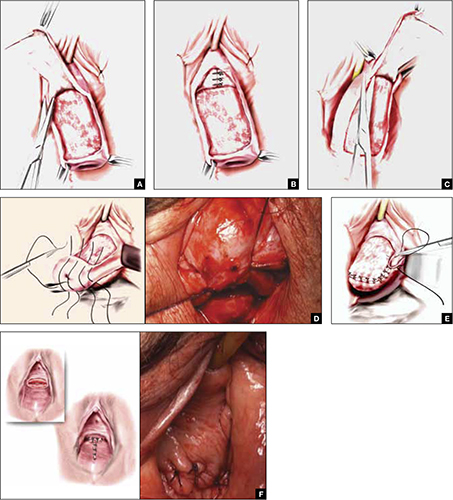
FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.
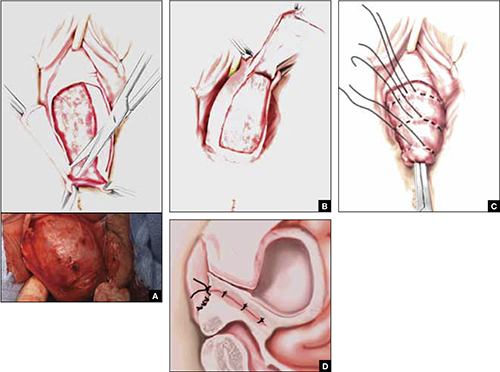
FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()
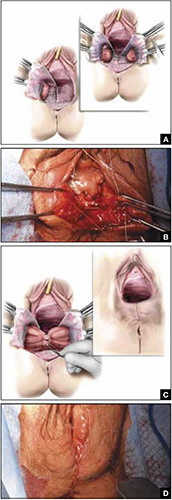
FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
High uterosacral vaginal vault suspension to repair enterocele and apical prolapse
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
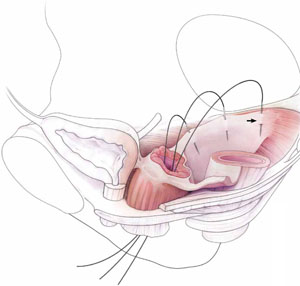
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).
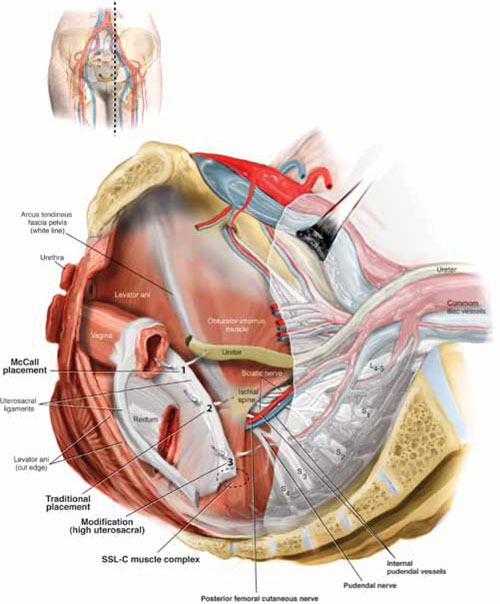
FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).
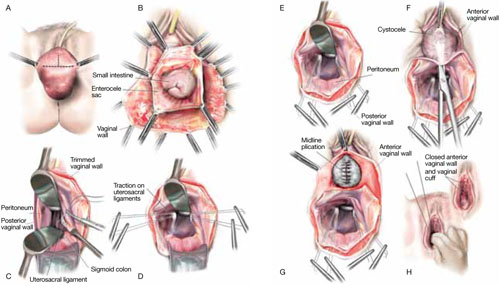
FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).
When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.
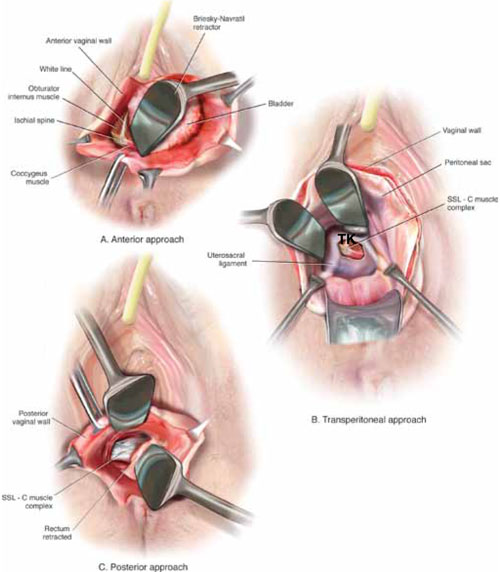
FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.
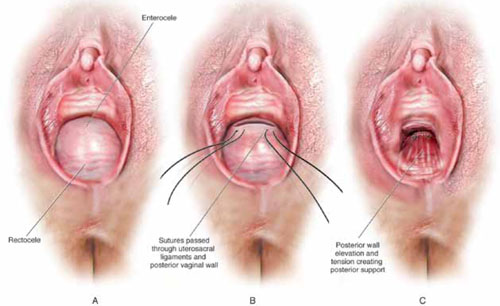
FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
- Pelvic anatomy of high intraperitoneal vaginal vault suspension
- Anatomy of the uterosacral ligament
- High uterosacral suspension (complete uterine procidentia)
- High uterosacral suspension (post-hysterectomy vaginal vault prolapse)
These videos were selected by Mickey Karram, MD, and Christine Vaccaro, DO, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS).
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
The concept of utilizing the uterosacral ligaments to support the vaginal cuff and correct an enterocele is nothing new: As early as 1957, Milton McCall described what became known as the McCall culdoplasty, in which sutures incorporated the uterosacral ligaments into the posterior vaginal vault to obliterate the cul-de-sac and suspend or support the vaginal apex at the time of vaginal hysterectomy.1
Later, in the 1990s, Richardson promoted the concept that, in patients who have pelvic organ prolapse, the uterosacral ligaments do not become attenuated, instead, they break at specific points.
Shull and colleagues took this idea and described how utilizing uterosacral ligaments to support the vaginal cuff can be performed vaginally—by passing sutures bilaterally through the uterosacral ligaments near the level of the ischial spine.2
Since Shull described this procedure, numerous published studies have demonstrated outcomes similar to other vaginal suspension procedures, such as sacrospinous ligament suspension.3-5
Potential advantages of a high uterosacral vaginal vault suspension are that:
- it provides good apical support without significantly distorting the vaginal axis, making it applicable to all types of vaginal prolapse
- intraperitoneal passage of sutures can be a lot cleaner and simpler than passing sutures, or anchors, through retroperitoneal structures, such as the sacrospinous ligament (FIGURE 1).

FIGURE 1 Locating intraperitoneal sutures during uterosacral suspension
Cross-section of the pelvic floor shows where sutures are placed as part of McCall culdoplasty (1), traditional uterosacral suspension (2), and modified high uterosacral suspension (3). Note: High uterosacral suspension may involve passing the suture through the sacrospinous ligament–coccygeus (SSL-C) muscle complex (dashed oval) because a segment of the uterosacral ligament inserts into that structure.
A disadvantage of the procedure is that the uterosacral ligament may, at times, lie in close proximity to the ureter. Studies have shown that the ureter can become kinked when sutures in this procedure are passed too far laterally.2-5
High uterosacral suspension has been our operation of choice for 11 years for patients who have pelvic organ prolapse in which the peritoneum is accessible (see “How this procedure evolved in our hands”). In this article, we provide a step-by-step description of the procedure. Four accompanying videos that further illuminate those steps are noted in the text here at appropriate places.(For example, Video #1, immediately below, sets the stage for the step-by-step discussion by reviewing pertinent pelvic anatomy.)
- When we first performed high uterosacral vaginal vault suspension as described by Shull and colleagues,1 we mobilized vaginal muscularis off the epithelium and suspended the epithelium and muscularis separately, making sure that sutures were passed through the anterior and Posterior vaginal walls.
- Initially, we thought that a large cul-de-sac needed to be obliterated in the midline with internal McCall-type stitches that were separate and distinct from the uterosacral suspension sutures. We no longer do this routinely because we believe that the numerous sutures that are passed through the full thickness of the posterior vaginal wall, including the peritoneum, effectively obliterate the enterocele and keep down the incidence of recurrent enterocele and high rectocele.
- We have come to realize that sutures placed medial and cephalad to the ischial spine are often passed through a portion of the coccygeus muscle-sacrospinous ligament complex. At times, a small window can be made in the peritoneum that provides direct access to this complex (FIGURE 1; FIGURE 3).
References
1. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
1. Enter the peritoneum
It’s our opinion that, even though extraperitoneal uterosacral suspension procedures have been described, the pertinent anatomic structures (again, see Video #1) are not easily identifiable unless suspension is undertaken intraperitoneally. Entering the peritoneum is, obviously, not a concern if the patient is undergoing vaginal hysterectomy. If the patient has post-hysterectomy prolapse, however, you must be able to isolate an enterocele and enter the peritoneum (follow FIGURE 2, beginning here and through subsequent steps of the procedure).

FIGURE 2 Step by step: High uterosacral vaginal vault suspension
A The most prominent portion of the prolapsed vaginal vault is grasped with two Allis clamps. B The vaginal wall is opened up and the enterocele sac is identified and entered. C The bowel is packed high into the pelvis using large laparotomy sponges. The retractor lifts the sponges out of the lower pelvis, thus completely exposing the cul-de-sac. When appropriate traction is placed downward on the uterosacral ligaments with an Allis clamp, the uterosacral ligaments are easily palpated bilaterally. D Delayed absorbable sutures have been passed through the uppermost portion of the uterosacral ligaments on each side, and have been individually tagged.
E Each end of the previously passed sutures is brought out through the posterior peritoneum and the posterior vaginal wall. (A free needle is used to pass both ends of these delayed absorbable sutures through the full thickness of the vaginal wall.) F Anterior colporrhaphy is begun by initiating dissection between the prolapsed bladder and the anterior vaginal wall. G Anterior colporrhaphy is complete. H The vagina has been appropriately trimmed and closed with interrupted or continuous delayed absorbable sutures. Delayed absorbable sutures that were previously brought out through the full thickness of the posterior vaginal wall are then tied; doing so elevates the prolapsed vaginal vault high up into the hollow of the sacrum.Once you have entered the peritoneum, the cul-de-sac must be relatively free of adhesive disease if you are to be able to continue with this procedure. (See “5 surgical pearls for high ureterosacral vaginal vault suspension”)
- Be prepared to convert to a sacrospinous fixation if you cannot enter the enterocele sac or if the posterior cul-de-sac is obliterated with adhesions
- Pass the sutures through durable tissue so that, when traction is placed on the sutures, there is minimal movement of peritoneum. Doing so might avoid kinking of the ureter.
- Pass the sutures through the full thickness of the posterior vaginal wall, including the peritoneum. Doing so not only suspends the apex but tremendously facilitates support for the posterior vaginal wall (FIGURE 4).
- When prolapse is very large, excise redundant portions of the upper part of the posterior vaginal wall and peritoneum—making sure, however, that you keep all layers together for performing the suspension. (See VIDEO #4, showing high uterosacral suspension in a patient who has complete uterine procidentia.)
- Do not try to pass a ureteral stent if you do not see indigo carmine dye spill from the ureteral orifices; to do so can be difficult after repair of prolapse, even in the hands of a skilled urologist. It is best instead to:
- identify the offending suture
- cut it
- visualize the spill of dye-colored urine
- proceed with either replacing the cut suture or maintaining the suspension with other, remaining sutures.
In our experience, when we have also performed an anterior repair, the ureter is kinked in at least 50% of cases because of one of the sutures that was used to correct the cystocele.
2. Pack the bowel; expose the uterosacral ligaments
Next, pack the small bowel out of the cul-de-sac to allow easy access and visualization of the uppermost portions of the uterosacral ligament. This is best accomplished by passing large, moistened laparotomy sponges intraperitoneally and elevating them with a large retractor (e.g., Deaver, Breisky-Navrital, Sweetheart).
When the bowel is appropriately packed, the retractor lifts the intestinal contents out of the pelvis, usually allowing easy access to the proximal or uppermost portion of the uterosacral ligaments (see Video #3, which focuses on the anatomy of the uterosacral ligament).

When performing high uterosacral suspension, it is possible to pass sutures through the coccygeus muscle-sacrospinous ligament complex (arrow) because a segment of the uterosacral ligament inserts into that structure.
3. Palpate the ischial spines bilaterally
It’s important that you palpate the ischial spines. Often, the ureter can be palpated against the pelvic sidewall. If you palpate the ischial spines and continue to palpate medially and cephalad, you can usually palpate the coccygeus muscle-sacrospinous ligament complex transperitoneally because a portion of the uterosacral ligament inserts into the sacrospinous ligament.6
If sutures can be passed at this level, the result will (usually) be a vagina that is, at minimum, approximately 9 cm long.

FIGURE 3 Access to the sacrospinous ligament
The sacrospinous ligament can be palpated and exposed along any one of three approaches: anterior paravaginally (A), transperitoneally (B), and posterior pararectally (C).
4. Pass the sutures
We prefer to pass two or three sutures on each side, utilizing a long, straight needle holder. Because we eventually pass the sutures through the full thickness of the posterior vaginal wall, we’ve opted for a delayed absorbable suture—preferably, 0 Vicryl on a CT-2 needle.
A Breisky-Navrital retractor is utilized to retract the sigmoid colon in the opposite direction of the ligament in which the sutures are being passed. At times, attaching a light to a suction device or a retractor is also helpful to visualize this area.
Use an Allis clamp to elevate and apply traction on the distal uterosacral ligament; this facilitates palpation and visualization of the appropriate site for placement of the sutures. The exact area of suture passage is best identified by palpation.
(Note: In early descriptions of this procedure, permanent sutures were utilized; again, we use delayed absorbable sutures because all sutures are brought out through the full thickness of the posterior vaginal wall. Permanent suture in our approach would be unacceptable because the sutures are tied in the lumen of the vagina. In some other modifications of this procedure, sutures are passed through the muscular layer of the vagina to exclude epithelium; under those circumstances, permanent sutures can be utilized.)
Once the sutures are brought through the full thickness of the posterior vaginal wall—including the peritoneum, if possible—tag them individually. If the anterior segment is well-supported, close the vaginal incision with a continuous delayed absorbable suture.
Tie the suspension sutures, elevating the apex into the hollow of the sacrum.
If anterior colporrhaphy is needed, perform that repair. Close the anterior vaginal wall as well as the vaginal cuff before tying off the suspension sutures.
5. Ensure that the ureters are patent
After the sutures are tied, instruct the anesthesiologist to administer 5 cc of indigo carmine dye intravenously. Assuming no renal compromise, you should see dye in the bladder 5 to 10 minutes later. If the patient is elderly or if you want to expedite this step, furosemide, 5 to 10 mg, can be given by IV push.
Next, perform cystoscopy to ensure ureteral patency. You should observe a spill of dye-colored urine out of both ureteral orifices. If dye does not spill from either orifice after a reasonable wait (usually, 20 minutes), assume that the ureter on that side is obstructed.

FIGURE 4 Providing support for the posterior vaginal wall
A View of a posterior vaginal wall defect secondary to an enterocele and rectocele. B After entry into the enterocele sac, intraperitoneal suspension sutures are brought out through the full thickness of the vaginal wall at the level of the apex. C Tying these sutures after the vaginal incision is closed at the apex not only results in greater vaginal length but also contributes to overall support of the entire posterior vaginal wall.
6. Completely reconstruct the vagina
The remainder of steps required to complete the procedure usually involve posterior colporrhaphy and perineoplasty. We also reserve placement of a synthetic midurethral sling (if one is needed) until after the vault procedure is complete.
Refer to FIGURE 2 for a step-by step guide to how best to perform high uterosacral vaginal vault suspension.
Questions often asked about this procedure
What do I do if I can’t isolate an enterocele sac and enter it?
Perform a unilateral or bilateral sacrospinous ligament colpopexy.
Is it always possible to identify a usable uterosacral ligament in patients who have advanced prolapse?
We’ve found it extremely rare not to be able identify a usable and durable structure.
The trick to identifying the ligament is to pass an Allis clamp so that one end is positioned intraperitoneally, as high up as possible, and the other end is on the vaginal mucosa side. Elevating the clamp puts the ligament on tension. These clamps are usually placed between 4 and 5 o’clock on the left side and between 7 and 8 o’clock on the right side.
With appropriate traction, the ligament can usually be easily palpated.
If I don’t see indigo carmine dye spilling from one side during cystoscopy, what sequence of events should I undertake?
If the only sutures placed on that side were the uterosacral ligament sutures, cut them individually. If the ureter spills dye after a suture is cut, decide whether you think it is appropriate to replace that suture. Sometimes, unilateral suspension or a suspension with one remaining suture on the side where you cut a suture or two is sufficient.
If you do want to replace a cut suture, ureteral patency must be confirmed again after it is replaced.
No further management of the ureter is required—that is, it isn’t necessary to catheterize the ureter or perform postoperative imaging studies. If anterior colporrhaphy has also been performed, however, apply your highest index of suspicion to determine the source of the offending suture: the uterosacral suspension or the anterior repair.*
If the patient has severe hip or leg pain postoperatively, what should I suspect is wrong? How should I manage this complication?
The nerve to the levator ani runs within the coccygeus muscle. In a thin patient, in whom deep bites are taken, the nerve is often injured or trapped. Such trauma can cause hip pain that is fairly severe but that is almost always self-limiting and requires only nonsteroidal anti-inflammatory medication. Usually, this complication resolves within 2 weeks after surgery.
Significant postoperative pain that radiates down the back of the thigh or down the leg all the way to the foot is of greater concern because one of the sacral nerve segments has most likely been injured or stretched. Obtain a neurology consult; rarely, it becomes necessary to take the patient back to surgery to cut the offending suture.
*For detailed discussion of this subject, see the International Academy of Pelvic Surgery’s August 2010 “Case of the month” at www.academyofpelvicsurgery.com.
We want to hear from you! Tell us what you think.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
1. McCall ML. Posterior culdeplasty; surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10(6):595-602.
2. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365-1374.
3. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183(6):1402-1411.
4. Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185(6):1339-1343.
5. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006;108(2):255-263.
6. Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;13(3):447-451.
Which sling for which patient?
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
Office evaluation of overactive bladder: 4 easy steps
A 66-year-old woman complains of urinary urgency, frequency, and incontinence, and estimates that she voids 15 or more times within a typical 24-hour period. So far, she has lost only small amounts of urine—because she hurries to void at the first sense of urgency—but she is distressed and worried that she will have a major accident.
Sound familiar? Overactive bladder affects 17 to 33 million US women.1 Thanks to greater awareness and openness, more women today are seeking medical help for their troubling symptoms, although only a fraction have done so up to now.2 Ob/Gyns who are prepared to quickly evaluate the problem and initiate effective management can help restore the quality of life these patients enjoyed before onset of symptoms. This article:
- reviews the pathophysiology of “overactive bladder”
- describes a 4-step evaluation and management routine that should be feasible for any gynecology office setting;
- discusses the action and the efficacy of available and forthcoming drugs;
- uses newly revised terminology that reflects greater sensitivity to the patient.
- Ask the right questions, get voiding diary, assess quality of life.
- Perform ‘eyeball’ cystometry.
- Conduct a thorough physical assessment.
- Begin bladder retraining, pelvic floor muscle rehabilitation, and appropriate medical therapy.
Revised terminology
One of the most notable changes in the terms used to describe lower urinary tract dysfunction, proposed by the International Continence Society,3 is organization of the terminology into 3 categories: symptoms, signs, and urodynamic observations.
Symptoms are now defined to more closely reflect the way the patient perceives her problem, and are set forth without specifying the volume of urine required for a diagnosis of “abnormal” sensation or urgency.
Signs can be observed by the physician, such as leakage of urine when the patient coughs.
Urodynamic observations are made during urodynamic studies.
Overall, the new and revised terms are relatively vague to allow for patient-to-patient variability. Here are a few examples:
- Overactive bladder is a syndrome of symptoms that suggest dysfunction of the lower urinary tract. It is characterized by urgency with or without urge incontinence, usually involving frequency and nocturia.
- Urinary incontinence is any involuntary leakage of urine.
- Daytime frequency. The patient feels she voids more frequently than she should during the day.
- Nocturia. The patient wakes 1 or more times at night to void.
- Urgency. The patient feels a sudden, compelling desire to pass urine.
- Urge urinary incontinence is involuntary leakage accompanied by or immediately preceded by urgency.
- Bladder sensation is identified during history taking: normal, increased, reduced, absent, and nonspecific.
- Detrusor overactivity replaces the term “detrusor instability” or “hyperreflexia.” It is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, and may be spontaneous or provoked. It may be further qualified as neurogenic (if a neurologic condition underlies the problem) or as idiopathic.
What is abnormal bladder function?
Any actual incontinence should be considered abnormal, whether diurnal or nocturnal.
Frequency: More than 8 voids in 24 hours. Although an ordinary voiding pattern is not fully defined, most experts agree that a frequency of 8 or fewer voids in 24 hours is “normal.”
Urgency: Patient’s opinion determines. The sensation of urgency is more difficult to objectively define; hence, the need to rely on the patient’s perceptions. If a patient is voiding more frequently than normal because she has an uncomfortable, sudden desire to pass urine, she is considered to have urgency. In contrast, a woman who voids frequently because she has stress incontinence and wants to keep her bladder as empty as possible to avoid leakage has frequency without urgency. Urgency is best classified as being sensory or motor in nature.
- Sensory urgency is a strong, uncomfortable need to void without fear of impending leakage; for whatever reason, the bladder has become hypersensitive. Delaying voiding may result in pain but rarely leads to incontinence.
- Patients with motor urgency urinate frequently because they are afraid of experiencing a complete or partial involuntary void as a result of an involuntary bladder contraction.
How the normal bladder functions
The process of bladder storage and evacuation can be visualized as a complex of neurocircuits in the brain and spinal cord that coordinate the activity of smooth muscle in the bladder and urethra (FIGURE). These circuits act as “on/off” switches in the lower urinary tract, alternating between the 2 modes of operation: storage and elimination.
As the bladder gradually fills with urine, a woman initially perceives a first sensation of filling between 75 and 125 cc of urine, feels the first need to void at approximately 300 cc, and reaches maximum capacity and a strong urge to void at 400 to 700 cc.
Since the bladder is a low-pressure reservoir, intravesical bladder pressure typically rises very little despite increasing amounts of urine and distention of the smooth muscle or detrusor muscle of the bladder. Pressure ranges from 2 to 6 cm of water in an empty state and rarely exceeds 10 cm of water at maximum capacity.
At maximum capacity, a woman should be able to get to the toilet easily, initiate voluntary bladder contraction with complete relaxation of her pelvic floor, and void to completion.
Urge incontinence is more detrimental to quality of life
Of women who complain of urinary incontinence, more than 90% have either loss of detrusor muscle control (urge incontinence) or urethral sphincteric incompetence (stress incontinence).4 In addition, 30% to 50% of women with stress incontinence have coexistent urge incontinence. 1
Urge incontinence has a much more dramatic impact on a woman’s quality of life than stress incontinence, because stress incontinence is predictable and controllable. The patient understands she will leak urine only with increases in intraabdominal pressure associated with exercise, coughing, etc. These leakages tend to occur in small spurts that are easily absorbed by protective wear. In contrast, urge incontinence manifests as an unpredictable, involuntary void in which urine is released in a gushing stream, often in quantities large enough to soak through heavy absorbent pads.
Although one might assume that subjective complaints would readily distinguish the 2 conditions, the bladder is a very poor “witness.” What the patient perceives often fails to correlate with the true mechanism of incontinence. Since therapies for these 2 conditions are completely different, the evaluation of incontinence is very important.
In aging women, the prevalence of frequency, urgency, and urge incontinence is much higher than that of stress incontinence. Among women 60 to 80 years of age—growth-wise, the largest segment of our population—as many as 50% experience frequency, urgency, and urge incontinence.
High economic cost. The tremendous expense of urinary incontinence is increasingly recognized. In 1995, for example, the economic cost in the United States was $26.3 billion, or $3,565 per person 65 years or older with the condition.5,6 Of these resources, 48%, or $12.53 billion, were drawn directly from the economy to diagnose, treat, care for, and rehabilitate patients with incontinence.
Contributing factors and causes of overactive bladder
Overactive bladder is thought to be multifactorial. Symptoms often occur in the absence of any obvious pathology, which makes it difficult to pinpoint a cause. Coexisting conditions may also contribute to symptoms or may even be the sole cause.
Examples include infection or inflammation of the lower urinary tract, such as a simple case of cystitis, or a foreign body in the bladder.
Injury or diseases of the nervous system can disrupt voluntary control of voiding in adults, triggering the reemergence of reflex voiding, which leads to bladder hyperactivity and urge incontinence. At a local level, urge incontinence can develop secondary to intrinsic detrusor myogenic abnormalities.
Outlet obstruction can result in urge incontinence such as the well recognized symptoms of urethral obstruction in men with benign prostatic hyperplasia.
Detrusor sphincter dysnergia, most commonly secondary to spinal cord injury or multiple sclerosis, may affect younger men and women.
A deficient urethral sphincter in women with stress incontinence may induce urge incontinence, as urine leaking into the urethra secondary to the stress incontinence stimulates urethral afferents that induce involuntary voiding reflexes.7
Women with stress incontinence may unwittingly contribute to overactive bladder by voiding more and more often, hoping to prevent any involuntary urine loss. As a result of the frequent voiding, they develop frequency and urgency symptoms. That is, over time, this frequent, voluntary voiding leads to decreased bladder compliance. Thus begins a vicious cycle that ultimately leads to more frequency and urgency.
Urogenital atrophy. Irritative symptoms of the lower urinary tract in the form of frequency, urgency, and dysuria can result from lack of estrogen, leading to urogenital atrophy.
Pelvic organ prolapse is another common coexisting condition. Although the correlation between anatomic descent of pelvic organs and lower urinary tract symptoms is poorly understood, frequency and urgency—with or without urge incontinence—coexist with symptomatic pelvic organ prolapse in approximately 30% to 50% of cases.
An enlarged uterus or adnexal mass may cause external compression of the bladder and lead to lower urinary tract symptoms.
Previous surgery of the anterior vaginal wall or bladder neck may sometimes trigger de novo symptoms of frequency, urgency, and urge incontinence. In women who have undergone a previous antiincontinence procedure, these symptoms may be related to some form of outlet obstruction. In some cases these patients have no increase in the postvoid residual, and only subtle urodynamic testing elicits evidence of obstruction.
Step 1Ask the right questions, get voiding diary, assess quality of life
Most women can be thoroughly evaluated within the clinical practice setting of any gynecologist. The first and most important aspect of this assessment is understanding and appreciating the severity of a patient’s lower urinary tract symptoms. This can be done by asking pointed questions, in the following approximate sequence:
- Do you have problems with accidental loss of urine?
- How many months or years have you had leakage?
- Do you have to wear pads or protective clothing to prevent or help with urinary loss? If so, how many pads do you wear a day?
- How many trips do you make to the bathroom during the day? At night?
- Do you ever wet the bed while sleeping?
- Are you bothered by a strong sense of urgency to void? Can you overcome it?
- Do you sometimes fail to reach the bathroom in time?
- Does the sound, sight, or feel of running water cause you to lose urine?
- Do you lose urine when you cough, sneeze, run, or lift heavy objects?
- Do you lose urine with posture changes, standing, or walking?
- Do you feel as though you are constantly wet?
- Do you feel as though your bladder is completely empty after passing urine?
- Do you have difficulty starting a stream of urine?
Take a thorough medical history, as well as a surgical history with emphasison previous bladder or gynecologic procedures.
Also review all prescription medications.
48-hour voiding diary. Give the patient a voiding diary to fill out 48 hours prior to her office visit. The reason: The diary often reveals more information than can be elicited from the patient’s history. For example, it may highlight daily activities associated with voiding, such as excessive consumption of liquids, high caffeine intake, high-impact exercise, and so on.
Quality-of-life assessment. An objective means of quantifying the effects of incontinence on the woman’s quality of life is recommended. We use the short form of the Incontinence Impact Questionnaire and the Urinary Distress Inventory.
Step 2Perform ‘eyeball’ cystometry, a simple and revealing office test
Ask the patient to go to the restroom and comfortably empty her bladder into a urine-collection device to determine the amount voided. Have a nurse measure the postvoid residual using a soft red rubber catheter. A sample can be taken for urinalysis and, if necessary, sent for culture.
Next, perform a simple filling or “eyeball” cystometry. Connect a Toomey syringe to the end of the red rubber catheter and pour sterile water into it. Ask the patient to tell you when she feels the first sensation of filling, first desire to void, strong urge to void, and maximum capacity, recording the levels at which each occurs. During filling, any evidence of bladder contraction will be revealed by a rise in the column of water. Record any significant discomfort or other observations during the filling portion of the study. When maximum capacity is reached, remove the catheter.
Step 3Conduct a thorough physical assessment
With the patient in the supine position, separate the labia and ask her to cough forcefully and perform the Valsalva maneuver 3 times, recording any evidence of water or urine loss through the urethral meatus. (If the patient has advanced pelvic organ prolapse, try to reduce the prolapse to eliminate any anatomic distortion of the urethra.)
Then ask the patient to stand with a full bladder and to squat, again having her cough forcefully 3 times. Record any additional urinary loss. Finally, ask the patient to void and again record the amount voided.
After the patient has emptied her bladder, again ask her to cough forcefully 3 times in the supine position, noting any evidence of leakage from the urethra (empty supine stress test).
Perform an overall inspection of the perineum and external genitalia and record a description in the patient’s chart.
Attempt to elicit an anal wink, and perform a brief neurological examination to ensure that spinal cord segments S2, S3, and S4 are intact. Next, gently insert a finger into the vagina and ask the patient to forcefully squeeze around it. Record the forcefulness of the squeeze on a scale of 0 to 5, with 0 being no appreciable movement and 5 being the most forceful squeeze possible. During this portion of the exam, instruct the patient on how to perform a Kegel exercise without recruiting the muscles of the buttocks and the abdominal wall.
Next, use a finger to gently massage the urethra, looking for any possible discharge from the urethral meatus that would be consistent with urethral diverticulum. Also note any tenderness or pain elicited during the exam.
Inserting a half-speculum into the vagina, displace the rectum away from the bladder. As the patient performs a Valsalva maneuver or coughs forcefully, evaluate the support of the anterior vaginal wall. Then turn the speculum 180 degrees and displace the bladder anteriorly, examining the posterior pelvic wall for any signs of prolapse. Also note any urogenital atrophy.
Finally, use a full speculum to evaluate the vaginal apex and cervix (if the patient has not had a hysterectomy). Bimanual and abdominal examinations also are important to rule out any abdominal or pelvic mass that could be irritating or causing pressure on the bladder.
Reexamine the patient for evidence of prolapse when she is standing. Some cases are difficult to detect when the patient is supine.
Step 4Begin multipronged therapy
Bladder retraining. Review the patient’s history, voiding diary, and findings from the physical exam to identify an appropriate treatment. After sharing all findings with the patient, instruct the patient on bladder retraining, which has 3 main components: education, scheduled voiding, and positive reinforcement. (Bladder retraining can be an extremely successful modality.)
Education consists of explaining the pathophysiology of overactive bladder and answering any questions the patient may have, so she understands why she is having the problem.
For scheduled voiding—also known as bladder retraining—examine the voiding diary to determine the approximate length of time between voids in a day. For instance, if the patient voids every hour, we generally ask her to continue doing so for the first week of retraining. The following week, we increase the interval to an hour and 15 minutes, the third week to 1.5 hours, and so on, with an ultimate goal of 3 hours between voids.
We also give instructions on pelvic floor rehabilitation or Kegel exercises. If the patient is able to voluntarily contract her pelvic floor muscles adequately, we recommend an exercise regimen that involves contracting her muscles numerous times a day for at least 10 seconds each time. If she is unable to contract her muscles or has a “dead” pelvic floor, she is referred to a physical therapist for biofeedback and possibly electrical stimulation.
We also instruct the patient to avoid racing to the bathroom when she feels an urge to void. Instead, have her stop, contract her pelvic floor muscles, and allow the urge to pass. She can then walk comfortably to the bathroom.
As mentioned earlier, the voiding diary may highlight problems such as excessive intake of fluids.
Local estrogen therapy can be added if there are signs of urogenital atrophy.
Medical therapy. In addition to education, timed voids, pelvic floor rehabilitation, and estrogen therapy, we usually start the extended-release form of tolterodine (4 mg) or oxybutynin (10 mg), which are antimuscarinic agents. (See “Treating overactive bladder: An expanding ‘pharmacopeia’”).
We ask her to return for a follow-up visit in 4 to 6 weeks to inquire about her symptoms and any significant side effects. We ask the patient to prepare another voiding diary for that visit so we can objectively measure decreases in frequency, urgency, and urge incontinence.
Dose adjustment is very important in patients with overactive bladder. If the woman is experiencing minimal effect with no side effects, titrate the dosage upward. If she is having good effect with significant side effects, decrease the dosage or consider switching to the transdermal form of oxybutynin.
When all these conditions are met and the patient remains severely affected, we perform multichannel urodynamic testing along with cystoscopy. If the testing supports the diagnosis of overactive bladder, and all other mechanisms for improvement have been exhausted, we counsel the patient about intravesical administration of botulism toxin, which relaxes skeletal and smooth muscle by preventing release of acetocholine from nerve terminal endings. Another option is sacroneuromodulation (InterStim therapy).
Antimuscarinic medications have been the mainstay of pharmacologic therapy for overactive bladder. In fact, the 2 most commonly prescribed drugs for overactive bladder are antimuscarinics: oxybutynin and tolterodine. Other medications also are available that affect different aspects of the neurophysiology of micturition.
Oxybutynin. The bladder contains 5 subtypes of muscarinic receptors, with a predominance of M2 and M3 subtypes. Oxybutynin has some selectivity for M3 receptors. It has been shown to inhibit bladder contractions, but because of muscarinic blockade in other parts of the body, it can have bothersome side effects. For example, oxybutynin has shown a higher affinity for muscarinic receptors in the parotid gland than the bladder, leading to dry mouth. In addition, the active metabolite of oxybutynin, N-Desethyl-oxybutynin, can reach concentrations that are 6 times the parent compound after oral administration.9 Other side effects include constipation, gastrointestinal upset, and blurry vision.
Alternate routes of administration have been developed to improve tolerability, including extended-release oral formulations, rectal administration, and a transdermal patch. Oxybutynin XL is the extended-release form, which incorporates a push-pull osmotic system. In parallel randomized, controlled clinical trials comparing immediate- and extended-release oxybutynin, oxybutynin XL was just as effective as the immediate-release formulation but had fewer side effects.10-12 Absorption of oxybutynin XL may occur to a larger degree in the colon than in the stomach and proximal small intestine, thereby decreasing conversion to N-Desethyl-oxybutynin. Oxybutynin XL comes in 3 doses (5, 10, and 15 mg) and offers the advantage of dose titration, a very important aspect of management.
A transdermal version of oxybutynin was recently released. In theory, transdermal treatment offers potential advantages such as more stable plasma concentrations and lower presystemic metabolism, which may decrease the primary active metabolite of N-Desethyl-oxybutynin. In a randomized, placebo-controlled trial of 3 doses of transdermal oxybutynin (1.3, 2.6, and 3.9 mg daily), only the highest dose out-performed placebo for median changes in incontinence episodes, frequency, and normal void volume.13 Quality of life also was significantly improved with the 3.9-mg dose. Anticholinergic side effects were comparable between active and placebo groups.
Tolterodine is a potent muscarinic-receptor antagonist without selectivity for particular subtypes. It is marketed in both immediate- and extended-release (tolterodine LA) formulations. A recent series compared both forms of the drug with placebo in 1,529 adults with overactive bladder.14 The primary measure of efficacy was change in the mean number of incontinent episodes weekly. Both medications showed a significantly better response than placebo. Dry mouth was significantly lower with tolterodine LA. When the authors used median values instead of mean values (because of non-normal distributed data), tolterodine LA showed improved efficacy over the immediate-release formulation. This study was sponsored by the drug’s manufacturer.
Comparisons of oxybutynin and tolterodine. In a prospective, double-blind, head-to-head study of the 2 drugs sponsored by the manufacturer of oxybutynin XL, 378 men and women were randomized to receive oxybutynin XL (10 mg daily) or tolterodine immediate-release (2 mg twice daily).15 Inclusion criteria included 7 or more episodes of urge incontinence per week and at least 10 voids per 24 hours. The Overactive Bladder: Judging Effective Control and Treatment (OBJECT) trial demonstrated greater efficacy with extended-release oxybutynin than with tolterodine, although both medications decreased weekly episodes of urge incontinence. Oxybutynin XL also showed a small but significant advantage for mixed incontinence and urinary frequency. Dry mouth and central nervous system side effects were similar for the 2 drugs.
A second head-to-head study also was sponsored by the manufacturer of oxybutynin—this one a randomized, double-blind comparison of the extended-release versions of both drugs. The Overactive Bladder: Performance of Extended Release Agents (OPERA) trial randomized 790 women to oxybutynin XL (10 mg daily) or tolterodine LA (4 mg daily).16 Inclusion criteria included at least 21 to 60 incontinent episodes per week and urgency and frequency exceeding 10 episodes per 24 hours. The drugs were similar in both measures, as well as in side effects, although dry mouth, usually mild, was more common among oxybutynin users. However, oxybutynin was significantly more effective in reducing micturition frequency, and 23% of women taking oxybutynin reported no episodes of incontinence, compared with 16.8% of women taking tolterodine. This finding was significant.
A third head-to-head study involved 2 separate trials conducted in parallel, with individual centers evaluating only 1 drug. In it, 1,289 patients were randomized to open-label treatment with either 2 mg or 4 mg of tolterodine LA in 1 trial and with 5 mg or 10 mg of oxybutynin XL in the other.17Inclusion criteria included 18 years of age or greater and symptoms of urinary urgency and frequency. After 8 weeks, tolterodine LA (4 mg) was found to be significantly more effective than either dose of oxybutynin XL, while tolterodine LA (2 mg) was similar to both doses of oxybutynin XL. This study was hampered by the fact that no data were supplied on the number of patients with isolated frequency and urgency versus those who had urge incontinence. Also, no objective parameters such as a voiding diary were utilized.
Three antimuscarinic drugs in the pipeline. Other antimuscarinic agents include trospium, a quaternary amine with some antimuscarinic activity. Because of its structure, it does not cross the brain barrier well and may thus have fewer central side effects.
Two new drugs awaiting approval from the US Food and Drug Administration are darifenacin, which has high selectivity for M3 receptors, and solifenacin, an M3 antagonist with greater selectivity for the bladder than the salivary glands in animal models.
All 3 drugs should be available in the United States some time in 2004.
Antiadrenergic medications. Because the bladder also contains alpha- and beta-adrenergic receptors, antiadrenergic medications have been developed. Currently available alpha-adrenergic agonists include ephedrine, phenyl-propanolamine, and pseudoephedrine. Beta-adrenergic agonists include isoproterenol and terbutaline.
Serotonergic agents. Both sympathetic and parasympathetic autonomic nuclei—as well as urethral sphincter motor nuclei—receive serotonergic input from the Raphe nuclei in the caudal brain stem. Serotonergic pathways generally enhance urine storage by activating sympathetic pathways and inhibiting parasympathetic pathways. Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor that may be effective against both urge and stress incontinence. It is currently awaiting approval by the US Food and Drug Administration.
Implications of the placebo effect
We lack a complete understanding of how all the parts of this process interact and why. Most of the medications in use affect only 1 part of the complex process that governs urine storage and elimination.
In controlled trials, patients on placebo have experienced 30% improvement—or greater—in their symptoms. This would seem to suggest that education, behavioral retraining, and attention from physicians are responsible for some of the improvement. Indeed, in a recent systematic review of 32 trials involving 6,800 participants, Herbison et al8 found significant but relatively small differences between anticholinergic medications and placebo for many of the outcomes studied. Obviously, we have more to learn about the intricacies of this condition before we can eliminate it completely.
1. Stewart W, Herzog R, Wein A, et al. Prevalence of overactive bladder in the US: results from the Noble Program. Poster presented at the Second International Consultation on Incontinence, July 2001, Paris, France.
2. Voelker R. International group seeks to dispel incontinence ‘taboo.’ JAMA. 1998;280:951.-
3. Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurol Urodynamics. 2002;21:167-178.
4. Walters MD, Diaz K. Q-tip test: a study of continent and incontinent women. Obstet Gynecol. 1987;70:208-211.
5. Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355.-
6. Wagner TH, Hu TW. Economic costs of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:127.-
7. Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204.-
8. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
9. Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate release oxybutynin. J Clin Pharmacol. 1999;39:289.-
10. Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999;161:1809.-
11. Birns J, Lukkari E, Malone-Lee JG. A randomized controlled trial comparing the efficacy of controlled-release oxybutynin tablets (10 mg once daily) with conventional oxybutynin tablets (5 mg twice daily) in patients whose symptoms were stabilized on 5-mg twice daily of oxybutynin. BJU Int. 2000;85:793.-
12. Versi E, Appell R, Mobley D, Patton W, Saltzstein D. Dry mouth with conventional and controlled released oxybutynin in urinary incontinence. The Ditropan XL Study Group. Obstet Gynecol. 2000;95:718.-
13. Dmochowski RR, Davila GW, Zinner NR, et al. Efficacy and safety of transdermal oxybutynin in patients with urge and mixed urinary incontinence. J Urol. 2002;168:580-586.
14. Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57:414.-
15. Appell RA, Sand P, Dmochowski R, et al. for the OBJECT Study Group Prospective randomized controlled trial of extended release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: results of the OBJECT Study. Mayo Clin Proc. 2001;76:358-363.
16. Diokno AC, Appell RA, Sand PK, et al. for the OPERA Study Group Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
17. Sussman D, Garely A. Treatment of overactive bladder with once-daily extended-release tolterodine or oxybutynin: the Antimuscarinic Clinical Effectiveness Trial (ACET). Curr Hosp Res Opin. 2002;18:177-184.
A 66-year-old woman complains of urinary urgency, frequency, and incontinence, and estimates that she voids 15 or more times within a typical 24-hour period. So far, she has lost only small amounts of urine—because she hurries to void at the first sense of urgency—but she is distressed and worried that she will have a major accident.
Sound familiar? Overactive bladder affects 17 to 33 million US women.1 Thanks to greater awareness and openness, more women today are seeking medical help for their troubling symptoms, although only a fraction have done so up to now.2 Ob/Gyns who are prepared to quickly evaluate the problem and initiate effective management can help restore the quality of life these patients enjoyed before onset of symptoms. This article:
- reviews the pathophysiology of “overactive bladder”
- describes a 4-step evaluation and management routine that should be feasible for any gynecology office setting;
- discusses the action and the efficacy of available and forthcoming drugs;
- uses newly revised terminology that reflects greater sensitivity to the patient.
- Ask the right questions, get voiding diary, assess quality of life.
- Perform ‘eyeball’ cystometry.
- Conduct a thorough physical assessment.
- Begin bladder retraining, pelvic floor muscle rehabilitation, and appropriate medical therapy.
Revised terminology
One of the most notable changes in the terms used to describe lower urinary tract dysfunction, proposed by the International Continence Society,3 is organization of the terminology into 3 categories: symptoms, signs, and urodynamic observations.
Symptoms are now defined to more closely reflect the way the patient perceives her problem, and are set forth without specifying the volume of urine required for a diagnosis of “abnormal” sensation or urgency.
Signs can be observed by the physician, such as leakage of urine when the patient coughs.
Urodynamic observations are made during urodynamic studies.
Overall, the new and revised terms are relatively vague to allow for patient-to-patient variability. Here are a few examples:
- Overactive bladder is a syndrome of symptoms that suggest dysfunction of the lower urinary tract. It is characterized by urgency with or without urge incontinence, usually involving frequency and nocturia.
- Urinary incontinence is any involuntary leakage of urine.
- Daytime frequency. The patient feels she voids more frequently than she should during the day.
- Nocturia. The patient wakes 1 or more times at night to void.
- Urgency. The patient feels a sudden, compelling desire to pass urine.
- Urge urinary incontinence is involuntary leakage accompanied by or immediately preceded by urgency.
- Bladder sensation is identified during history taking: normal, increased, reduced, absent, and nonspecific.
- Detrusor overactivity replaces the term “detrusor instability” or “hyperreflexia.” It is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, and may be spontaneous or provoked. It may be further qualified as neurogenic (if a neurologic condition underlies the problem) or as idiopathic.
What is abnormal bladder function?
Any actual incontinence should be considered abnormal, whether diurnal or nocturnal.
Frequency: More than 8 voids in 24 hours. Although an ordinary voiding pattern is not fully defined, most experts agree that a frequency of 8 or fewer voids in 24 hours is “normal.”
Urgency: Patient’s opinion determines. The sensation of urgency is more difficult to objectively define; hence, the need to rely on the patient’s perceptions. If a patient is voiding more frequently than normal because she has an uncomfortable, sudden desire to pass urine, she is considered to have urgency. In contrast, a woman who voids frequently because she has stress incontinence and wants to keep her bladder as empty as possible to avoid leakage has frequency without urgency. Urgency is best classified as being sensory or motor in nature.
- Sensory urgency is a strong, uncomfortable need to void without fear of impending leakage; for whatever reason, the bladder has become hypersensitive. Delaying voiding may result in pain but rarely leads to incontinence.
- Patients with motor urgency urinate frequently because they are afraid of experiencing a complete or partial involuntary void as a result of an involuntary bladder contraction.
How the normal bladder functions
The process of bladder storage and evacuation can be visualized as a complex of neurocircuits in the brain and spinal cord that coordinate the activity of smooth muscle in the bladder and urethra (FIGURE). These circuits act as “on/off” switches in the lower urinary tract, alternating between the 2 modes of operation: storage and elimination.
As the bladder gradually fills with urine, a woman initially perceives a first sensation of filling between 75 and 125 cc of urine, feels the first need to void at approximately 300 cc, and reaches maximum capacity and a strong urge to void at 400 to 700 cc.
Since the bladder is a low-pressure reservoir, intravesical bladder pressure typically rises very little despite increasing amounts of urine and distention of the smooth muscle or detrusor muscle of the bladder. Pressure ranges from 2 to 6 cm of water in an empty state and rarely exceeds 10 cm of water at maximum capacity.
At maximum capacity, a woman should be able to get to the toilet easily, initiate voluntary bladder contraction with complete relaxation of her pelvic floor, and void to completion.
Urge incontinence is more detrimental to quality of life
Of women who complain of urinary incontinence, more than 90% have either loss of detrusor muscle control (urge incontinence) or urethral sphincteric incompetence (stress incontinence).4 In addition, 30% to 50% of women with stress incontinence have coexistent urge incontinence. 1
Urge incontinence has a much more dramatic impact on a woman’s quality of life than stress incontinence, because stress incontinence is predictable and controllable. The patient understands she will leak urine only with increases in intraabdominal pressure associated with exercise, coughing, etc. These leakages tend to occur in small spurts that are easily absorbed by protective wear. In contrast, urge incontinence manifests as an unpredictable, involuntary void in which urine is released in a gushing stream, often in quantities large enough to soak through heavy absorbent pads.
Although one might assume that subjective complaints would readily distinguish the 2 conditions, the bladder is a very poor “witness.” What the patient perceives often fails to correlate with the true mechanism of incontinence. Since therapies for these 2 conditions are completely different, the evaluation of incontinence is very important.
In aging women, the prevalence of frequency, urgency, and urge incontinence is much higher than that of stress incontinence. Among women 60 to 80 years of age—growth-wise, the largest segment of our population—as many as 50% experience frequency, urgency, and urge incontinence.
High economic cost. The tremendous expense of urinary incontinence is increasingly recognized. In 1995, for example, the economic cost in the United States was $26.3 billion, or $3,565 per person 65 years or older with the condition.5,6 Of these resources, 48%, or $12.53 billion, were drawn directly from the economy to diagnose, treat, care for, and rehabilitate patients with incontinence.
Contributing factors and causes of overactive bladder
Overactive bladder is thought to be multifactorial. Symptoms often occur in the absence of any obvious pathology, which makes it difficult to pinpoint a cause. Coexisting conditions may also contribute to symptoms or may even be the sole cause.
Examples include infection or inflammation of the lower urinary tract, such as a simple case of cystitis, or a foreign body in the bladder.
Injury or diseases of the nervous system can disrupt voluntary control of voiding in adults, triggering the reemergence of reflex voiding, which leads to bladder hyperactivity and urge incontinence. At a local level, urge incontinence can develop secondary to intrinsic detrusor myogenic abnormalities.
Outlet obstruction can result in urge incontinence such as the well recognized symptoms of urethral obstruction in men with benign prostatic hyperplasia.
Detrusor sphincter dysnergia, most commonly secondary to spinal cord injury or multiple sclerosis, may affect younger men and women.
A deficient urethral sphincter in women with stress incontinence may induce urge incontinence, as urine leaking into the urethra secondary to the stress incontinence stimulates urethral afferents that induce involuntary voiding reflexes.7
Women with stress incontinence may unwittingly contribute to overactive bladder by voiding more and more often, hoping to prevent any involuntary urine loss. As a result of the frequent voiding, they develop frequency and urgency symptoms. That is, over time, this frequent, voluntary voiding leads to decreased bladder compliance. Thus begins a vicious cycle that ultimately leads to more frequency and urgency.
Urogenital atrophy. Irritative symptoms of the lower urinary tract in the form of frequency, urgency, and dysuria can result from lack of estrogen, leading to urogenital atrophy.
Pelvic organ prolapse is another common coexisting condition. Although the correlation between anatomic descent of pelvic organs and lower urinary tract symptoms is poorly understood, frequency and urgency—with or without urge incontinence—coexist with symptomatic pelvic organ prolapse in approximately 30% to 50% of cases.
An enlarged uterus or adnexal mass may cause external compression of the bladder and lead to lower urinary tract symptoms.
Previous surgery of the anterior vaginal wall or bladder neck may sometimes trigger de novo symptoms of frequency, urgency, and urge incontinence. In women who have undergone a previous antiincontinence procedure, these symptoms may be related to some form of outlet obstruction. In some cases these patients have no increase in the postvoid residual, and only subtle urodynamic testing elicits evidence of obstruction.
Step 1Ask the right questions, get voiding diary, assess quality of life
Most women can be thoroughly evaluated within the clinical practice setting of any gynecologist. The first and most important aspect of this assessment is understanding and appreciating the severity of a patient’s lower urinary tract symptoms. This can be done by asking pointed questions, in the following approximate sequence:
- Do you have problems with accidental loss of urine?
- How many months or years have you had leakage?
- Do you have to wear pads or protective clothing to prevent or help with urinary loss? If so, how many pads do you wear a day?
- How many trips do you make to the bathroom during the day? At night?
- Do you ever wet the bed while sleeping?
- Are you bothered by a strong sense of urgency to void? Can you overcome it?
- Do you sometimes fail to reach the bathroom in time?
- Does the sound, sight, or feel of running water cause you to lose urine?
- Do you lose urine when you cough, sneeze, run, or lift heavy objects?
- Do you lose urine with posture changes, standing, or walking?
- Do you feel as though you are constantly wet?
- Do you feel as though your bladder is completely empty after passing urine?
- Do you have difficulty starting a stream of urine?
Take a thorough medical history, as well as a surgical history with emphasison previous bladder or gynecologic procedures.
Also review all prescription medications.
48-hour voiding diary. Give the patient a voiding diary to fill out 48 hours prior to her office visit. The reason: The diary often reveals more information than can be elicited from the patient’s history. For example, it may highlight daily activities associated with voiding, such as excessive consumption of liquids, high caffeine intake, high-impact exercise, and so on.
Quality-of-life assessment. An objective means of quantifying the effects of incontinence on the woman’s quality of life is recommended. We use the short form of the Incontinence Impact Questionnaire and the Urinary Distress Inventory.
Step 2Perform ‘eyeball’ cystometry, a simple and revealing office test
Ask the patient to go to the restroom and comfortably empty her bladder into a urine-collection device to determine the amount voided. Have a nurse measure the postvoid residual using a soft red rubber catheter. A sample can be taken for urinalysis and, if necessary, sent for culture.
Next, perform a simple filling or “eyeball” cystometry. Connect a Toomey syringe to the end of the red rubber catheter and pour sterile water into it. Ask the patient to tell you when she feels the first sensation of filling, first desire to void, strong urge to void, and maximum capacity, recording the levels at which each occurs. During filling, any evidence of bladder contraction will be revealed by a rise in the column of water. Record any significant discomfort or other observations during the filling portion of the study. When maximum capacity is reached, remove the catheter.
Step 3Conduct a thorough physical assessment
With the patient in the supine position, separate the labia and ask her to cough forcefully and perform the Valsalva maneuver 3 times, recording any evidence of water or urine loss through the urethral meatus. (If the patient has advanced pelvic organ prolapse, try to reduce the prolapse to eliminate any anatomic distortion of the urethra.)
Then ask the patient to stand with a full bladder and to squat, again having her cough forcefully 3 times. Record any additional urinary loss. Finally, ask the patient to void and again record the amount voided.
After the patient has emptied her bladder, again ask her to cough forcefully 3 times in the supine position, noting any evidence of leakage from the urethra (empty supine stress test).
Perform an overall inspection of the perineum and external genitalia and record a description in the patient’s chart.
Attempt to elicit an anal wink, and perform a brief neurological examination to ensure that spinal cord segments S2, S3, and S4 are intact. Next, gently insert a finger into the vagina and ask the patient to forcefully squeeze around it. Record the forcefulness of the squeeze on a scale of 0 to 5, with 0 being no appreciable movement and 5 being the most forceful squeeze possible. During this portion of the exam, instruct the patient on how to perform a Kegel exercise without recruiting the muscles of the buttocks and the abdominal wall.
Next, use a finger to gently massage the urethra, looking for any possible discharge from the urethral meatus that would be consistent with urethral diverticulum. Also note any tenderness or pain elicited during the exam.
Inserting a half-speculum into the vagina, displace the rectum away from the bladder. As the patient performs a Valsalva maneuver or coughs forcefully, evaluate the support of the anterior vaginal wall. Then turn the speculum 180 degrees and displace the bladder anteriorly, examining the posterior pelvic wall for any signs of prolapse. Also note any urogenital atrophy.
Finally, use a full speculum to evaluate the vaginal apex and cervix (if the patient has not had a hysterectomy). Bimanual and abdominal examinations also are important to rule out any abdominal or pelvic mass that could be irritating or causing pressure on the bladder.
Reexamine the patient for evidence of prolapse when she is standing. Some cases are difficult to detect when the patient is supine.
Step 4Begin multipronged therapy
Bladder retraining. Review the patient’s history, voiding diary, and findings from the physical exam to identify an appropriate treatment. After sharing all findings with the patient, instruct the patient on bladder retraining, which has 3 main components: education, scheduled voiding, and positive reinforcement. (Bladder retraining can be an extremely successful modality.)
Education consists of explaining the pathophysiology of overactive bladder and answering any questions the patient may have, so she understands why she is having the problem.
For scheduled voiding—also known as bladder retraining—examine the voiding diary to determine the approximate length of time between voids in a day. For instance, if the patient voids every hour, we generally ask her to continue doing so for the first week of retraining. The following week, we increase the interval to an hour and 15 minutes, the third week to 1.5 hours, and so on, with an ultimate goal of 3 hours between voids.
We also give instructions on pelvic floor rehabilitation or Kegel exercises. If the patient is able to voluntarily contract her pelvic floor muscles adequately, we recommend an exercise regimen that involves contracting her muscles numerous times a day for at least 10 seconds each time. If she is unable to contract her muscles or has a “dead” pelvic floor, she is referred to a physical therapist for biofeedback and possibly electrical stimulation.
We also instruct the patient to avoid racing to the bathroom when she feels an urge to void. Instead, have her stop, contract her pelvic floor muscles, and allow the urge to pass. She can then walk comfortably to the bathroom.
As mentioned earlier, the voiding diary may highlight problems such as excessive intake of fluids.
Local estrogen therapy can be added if there are signs of urogenital atrophy.
Medical therapy. In addition to education, timed voids, pelvic floor rehabilitation, and estrogen therapy, we usually start the extended-release form of tolterodine (4 mg) or oxybutynin (10 mg), which are antimuscarinic agents. (See “Treating overactive bladder: An expanding ‘pharmacopeia’”).
We ask her to return for a follow-up visit in 4 to 6 weeks to inquire about her symptoms and any significant side effects. We ask the patient to prepare another voiding diary for that visit so we can objectively measure decreases in frequency, urgency, and urge incontinence.
Dose adjustment is very important in patients with overactive bladder. If the woman is experiencing minimal effect with no side effects, titrate the dosage upward. If she is having good effect with significant side effects, decrease the dosage or consider switching to the transdermal form of oxybutynin.
When all these conditions are met and the patient remains severely affected, we perform multichannel urodynamic testing along with cystoscopy. If the testing supports the diagnosis of overactive bladder, and all other mechanisms for improvement have been exhausted, we counsel the patient about intravesical administration of botulism toxin, which relaxes skeletal and smooth muscle by preventing release of acetocholine from nerve terminal endings. Another option is sacroneuromodulation (InterStim therapy).
Antimuscarinic medications have been the mainstay of pharmacologic therapy for overactive bladder. In fact, the 2 most commonly prescribed drugs for overactive bladder are antimuscarinics: oxybutynin and tolterodine. Other medications also are available that affect different aspects of the neurophysiology of micturition.
Oxybutynin. The bladder contains 5 subtypes of muscarinic receptors, with a predominance of M2 and M3 subtypes. Oxybutynin has some selectivity for M3 receptors. It has been shown to inhibit bladder contractions, but because of muscarinic blockade in other parts of the body, it can have bothersome side effects. For example, oxybutynin has shown a higher affinity for muscarinic receptors in the parotid gland than the bladder, leading to dry mouth. In addition, the active metabolite of oxybutynin, N-Desethyl-oxybutynin, can reach concentrations that are 6 times the parent compound after oral administration.9 Other side effects include constipation, gastrointestinal upset, and blurry vision.
Alternate routes of administration have been developed to improve tolerability, including extended-release oral formulations, rectal administration, and a transdermal patch. Oxybutynin XL is the extended-release form, which incorporates a push-pull osmotic system. In parallel randomized, controlled clinical trials comparing immediate- and extended-release oxybutynin, oxybutynin XL was just as effective as the immediate-release formulation but had fewer side effects.10-12 Absorption of oxybutynin XL may occur to a larger degree in the colon than in the stomach and proximal small intestine, thereby decreasing conversion to N-Desethyl-oxybutynin. Oxybutynin XL comes in 3 doses (5, 10, and 15 mg) and offers the advantage of dose titration, a very important aspect of management.
A transdermal version of oxybutynin was recently released. In theory, transdermal treatment offers potential advantages such as more stable plasma concentrations and lower presystemic metabolism, which may decrease the primary active metabolite of N-Desethyl-oxybutynin. In a randomized, placebo-controlled trial of 3 doses of transdermal oxybutynin (1.3, 2.6, and 3.9 mg daily), only the highest dose out-performed placebo for median changes in incontinence episodes, frequency, and normal void volume.13 Quality of life also was significantly improved with the 3.9-mg dose. Anticholinergic side effects were comparable between active and placebo groups.
Tolterodine is a potent muscarinic-receptor antagonist without selectivity for particular subtypes. It is marketed in both immediate- and extended-release (tolterodine LA) formulations. A recent series compared both forms of the drug with placebo in 1,529 adults with overactive bladder.14 The primary measure of efficacy was change in the mean number of incontinent episodes weekly. Both medications showed a significantly better response than placebo. Dry mouth was significantly lower with tolterodine LA. When the authors used median values instead of mean values (because of non-normal distributed data), tolterodine LA showed improved efficacy over the immediate-release formulation. This study was sponsored by the drug’s manufacturer.
Comparisons of oxybutynin and tolterodine. In a prospective, double-blind, head-to-head study of the 2 drugs sponsored by the manufacturer of oxybutynin XL, 378 men and women were randomized to receive oxybutynin XL (10 mg daily) or tolterodine immediate-release (2 mg twice daily).15 Inclusion criteria included 7 or more episodes of urge incontinence per week and at least 10 voids per 24 hours. The Overactive Bladder: Judging Effective Control and Treatment (OBJECT) trial demonstrated greater efficacy with extended-release oxybutynin than with tolterodine, although both medications decreased weekly episodes of urge incontinence. Oxybutynin XL also showed a small but significant advantage for mixed incontinence and urinary frequency. Dry mouth and central nervous system side effects were similar for the 2 drugs.
A second head-to-head study also was sponsored by the manufacturer of oxybutynin—this one a randomized, double-blind comparison of the extended-release versions of both drugs. The Overactive Bladder: Performance of Extended Release Agents (OPERA) trial randomized 790 women to oxybutynin XL (10 mg daily) or tolterodine LA (4 mg daily).16 Inclusion criteria included at least 21 to 60 incontinent episodes per week and urgency and frequency exceeding 10 episodes per 24 hours. The drugs were similar in both measures, as well as in side effects, although dry mouth, usually mild, was more common among oxybutynin users. However, oxybutynin was significantly more effective in reducing micturition frequency, and 23% of women taking oxybutynin reported no episodes of incontinence, compared with 16.8% of women taking tolterodine. This finding was significant.
A third head-to-head study involved 2 separate trials conducted in parallel, with individual centers evaluating only 1 drug. In it, 1,289 patients were randomized to open-label treatment with either 2 mg or 4 mg of tolterodine LA in 1 trial and with 5 mg or 10 mg of oxybutynin XL in the other.17Inclusion criteria included 18 years of age or greater and symptoms of urinary urgency and frequency. After 8 weeks, tolterodine LA (4 mg) was found to be significantly more effective than either dose of oxybutynin XL, while tolterodine LA (2 mg) was similar to both doses of oxybutynin XL. This study was hampered by the fact that no data were supplied on the number of patients with isolated frequency and urgency versus those who had urge incontinence. Also, no objective parameters such as a voiding diary were utilized.
Three antimuscarinic drugs in the pipeline. Other antimuscarinic agents include trospium, a quaternary amine with some antimuscarinic activity. Because of its structure, it does not cross the brain barrier well and may thus have fewer central side effects.
Two new drugs awaiting approval from the US Food and Drug Administration are darifenacin, which has high selectivity for M3 receptors, and solifenacin, an M3 antagonist with greater selectivity for the bladder than the salivary glands in animal models.
All 3 drugs should be available in the United States some time in 2004.
Antiadrenergic medications. Because the bladder also contains alpha- and beta-adrenergic receptors, antiadrenergic medications have been developed. Currently available alpha-adrenergic agonists include ephedrine, phenyl-propanolamine, and pseudoephedrine. Beta-adrenergic agonists include isoproterenol and terbutaline.
Serotonergic agents. Both sympathetic and parasympathetic autonomic nuclei—as well as urethral sphincter motor nuclei—receive serotonergic input from the Raphe nuclei in the caudal brain stem. Serotonergic pathways generally enhance urine storage by activating sympathetic pathways and inhibiting parasympathetic pathways. Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor that may be effective against both urge and stress incontinence. It is currently awaiting approval by the US Food and Drug Administration.
Implications of the placebo effect
We lack a complete understanding of how all the parts of this process interact and why. Most of the medications in use affect only 1 part of the complex process that governs urine storage and elimination.
In controlled trials, patients on placebo have experienced 30% improvement—or greater—in their symptoms. This would seem to suggest that education, behavioral retraining, and attention from physicians are responsible for some of the improvement. Indeed, in a recent systematic review of 32 trials involving 6,800 participants, Herbison et al8 found significant but relatively small differences between anticholinergic medications and placebo for many of the outcomes studied. Obviously, we have more to learn about the intricacies of this condition before we can eliminate it completely.
A 66-year-old woman complains of urinary urgency, frequency, and incontinence, and estimates that she voids 15 or more times within a typical 24-hour period. So far, she has lost only small amounts of urine—because she hurries to void at the first sense of urgency—but she is distressed and worried that she will have a major accident.
Sound familiar? Overactive bladder affects 17 to 33 million US women.1 Thanks to greater awareness and openness, more women today are seeking medical help for their troubling symptoms, although only a fraction have done so up to now.2 Ob/Gyns who are prepared to quickly evaluate the problem and initiate effective management can help restore the quality of life these patients enjoyed before onset of symptoms. This article:
- reviews the pathophysiology of “overactive bladder”
- describes a 4-step evaluation and management routine that should be feasible for any gynecology office setting;
- discusses the action and the efficacy of available and forthcoming drugs;
- uses newly revised terminology that reflects greater sensitivity to the patient.
- Ask the right questions, get voiding diary, assess quality of life.
- Perform ‘eyeball’ cystometry.
- Conduct a thorough physical assessment.
- Begin bladder retraining, pelvic floor muscle rehabilitation, and appropriate medical therapy.
Revised terminology
One of the most notable changes in the terms used to describe lower urinary tract dysfunction, proposed by the International Continence Society,3 is organization of the terminology into 3 categories: symptoms, signs, and urodynamic observations.
Symptoms are now defined to more closely reflect the way the patient perceives her problem, and are set forth without specifying the volume of urine required for a diagnosis of “abnormal” sensation or urgency.
Signs can be observed by the physician, such as leakage of urine when the patient coughs.
Urodynamic observations are made during urodynamic studies.
Overall, the new and revised terms are relatively vague to allow for patient-to-patient variability. Here are a few examples:
- Overactive bladder is a syndrome of symptoms that suggest dysfunction of the lower urinary tract. It is characterized by urgency with or without urge incontinence, usually involving frequency and nocturia.
- Urinary incontinence is any involuntary leakage of urine.
- Daytime frequency. The patient feels she voids more frequently than she should during the day.
- Nocturia. The patient wakes 1 or more times at night to void.
- Urgency. The patient feels a sudden, compelling desire to pass urine.
- Urge urinary incontinence is involuntary leakage accompanied by or immediately preceded by urgency.
- Bladder sensation is identified during history taking: normal, increased, reduced, absent, and nonspecific.
- Detrusor overactivity replaces the term “detrusor instability” or “hyperreflexia.” It is a urodynamic observation characterized by involuntary detrusor contractions during the filling phase, and may be spontaneous or provoked. It may be further qualified as neurogenic (if a neurologic condition underlies the problem) or as idiopathic.
What is abnormal bladder function?
Any actual incontinence should be considered abnormal, whether diurnal or nocturnal.
Frequency: More than 8 voids in 24 hours. Although an ordinary voiding pattern is not fully defined, most experts agree that a frequency of 8 or fewer voids in 24 hours is “normal.”
Urgency: Patient’s opinion determines. The sensation of urgency is more difficult to objectively define; hence, the need to rely on the patient’s perceptions. If a patient is voiding more frequently than normal because she has an uncomfortable, sudden desire to pass urine, she is considered to have urgency. In contrast, a woman who voids frequently because she has stress incontinence and wants to keep her bladder as empty as possible to avoid leakage has frequency without urgency. Urgency is best classified as being sensory or motor in nature.
- Sensory urgency is a strong, uncomfortable need to void without fear of impending leakage; for whatever reason, the bladder has become hypersensitive. Delaying voiding may result in pain but rarely leads to incontinence.
- Patients with motor urgency urinate frequently because they are afraid of experiencing a complete or partial involuntary void as a result of an involuntary bladder contraction.
How the normal bladder functions
The process of bladder storage and evacuation can be visualized as a complex of neurocircuits in the brain and spinal cord that coordinate the activity of smooth muscle in the bladder and urethra (FIGURE). These circuits act as “on/off” switches in the lower urinary tract, alternating between the 2 modes of operation: storage and elimination.
As the bladder gradually fills with urine, a woman initially perceives a first sensation of filling between 75 and 125 cc of urine, feels the first need to void at approximately 300 cc, and reaches maximum capacity and a strong urge to void at 400 to 700 cc.
Since the bladder is a low-pressure reservoir, intravesical bladder pressure typically rises very little despite increasing amounts of urine and distention of the smooth muscle or detrusor muscle of the bladder. Pressure ranges from 2 to 6 cm of water in an empty state and rarely exceeds 10 cm of water at maximum capacity.
At maximum capacity, a woman should be able to get to the toilet easily, initiate voluntary bladder contraction with complete relaxation of her pelvic floor, and void to completion.
Urge incontinence is more detrimental to quality of life
Of women who complain of urinary incontinence, more than 90% have either loss of detrusor muscle control (urge incontinence) or urethral sphincteric incompetence (stress incontinence).4 In addition, 30% to 50% of women with stress incontinence have coexistent urge incontinence. 1
Urge incontinence has a much more dramatic impact on a woman’s quality of life than stress incontinence, because stress incontinence is predictable and controllable. The patient understands she will leak urine only with increases in intraabdominal pressure associated with exercise, coughing, etc. These leakages tend to occur in small spurts that are easily absorbed by protective wear. In contrast, urge incontinence manifests as an unpredictable, involuntary void in which urine is released in a gushing stream, often in quantities large enough to soak through heavy absorbent pads.
Although one might assume that subjective complaints would readily distinguish the 2 conditions, the bladder is a very poor “witness.” What the patient perceives often fails to correlate with the true mechanism of incontinence. Since therapies for these 2 conditions are completely different, the evaluation of incontinence is very important.
In aging women, the prevalence of frequency, urgency, and urge incontinence is much higher than that of stress incontinence. Among women 60 to 80 years of age—growth-wise, the largest segment of our population—as many as 50% experience frequency, urgency, and urge incontinence.
High economic cost. The tremendous expense of urinary incontinence is increasingly recognized. In 1995, for example, the economic cost in the United States was $26.3 billion, or $3,565 per person 65 years or older with the condition.5,6 Of these resources, 48%, or $12.53 billion, were drawn directly from the economy to diagnose, treat, care for, and rehabilitate patients with incontinence.
Contributing factors and causes of overactive bladder
Overactive bladder is thought to be multifactorial. Symptoms often occur in the absence of any obvious pathology, which makes it difficult to pinpoint a cause. Coexisting conditions may also contribute to symptoms or may even be the sole cause.
Examples include infection or inflammation of the lower urinary tract, such as a simple case of cystitis, or a foreign body in the bladder.
Injury or diseases of the nervous system can disrupt voluntary control of voiding in adults, triggering the reemergence of reflex voiding, which leads to bladder hyperactivity and urge incontinence. At a local level, urge incontinence can develop secondary to intrinsic detrusor myogenic abnormalities.
Outlet obstruction can result in urge incontinence such as the well recognized symptoms of urethral obstruction in men with benign prostatic hyperplasia.
Detrusor sphincter dysnergia, most commonly secondary to spinal cord injury or multiple sclerosis, may affect younger men and women.
A deficient urethral sphincter in women with stress incontinence may induce urge incontinence, as urine leaking into the urethra secondary to the stress incontinence stimulates urethral afferents that induce involuntary voiding reflexes.7
Women with stress incontinence may unwittingly contribute to overactive bladder by voiding more and more often, hoping to prevent any involuntary urine loss. As a result of the frequent voiding, they develop frequency and urgency symptoms. That is, over time, this frequent, voluntary voiding leads to decreased bladder compliance. Thus begins a vicious cycle that ultimately leads to more frequency and urgency.
Urogenital atrophy. Irritative symptoms of the lower urinary tract in the form of frequency, urgency, and dysuria can result from lack of estrogen, leading to urogenital atrophy.
Pelvic organ prolapse is another common coexisting condition. Although the correlation between anatomic descent of pelvic organs and lower urinary tract symptoms is poorly understood, frequency and urgency—with or without urge incontinence—coexist with symptomatic pelvic organ prolapse in approximately 30% to 50% of cases.
An enlarged uterus or adnexal mass may cause external compression of the bladder and lead to lower urinary tract symptoms.
Previous surgery of the anterior vaginal wall or bladder neck may sometimes trigger de novo symptoms of frequency, urgency, and urge incontinence. In women who have undergone a previous antiincontinence procedure, these symptoms may be related to some form of outlet obstruction. In some cases these patients have no increase in the postvoid residual, and only subtle urodynamic testing elicits evidence of obstruction.
Step 1Ask the right questions, get voiding diary, assess quality of life
Most women can be thoroughly evaluated within the clinical practice setting of any gynecologist. The first and most important aspect of this assessment is understanding and appreciating the severity of a patient’s lower urinary tract symptoms. This can be done by asking pointed questions, in the following approximate sequence:
- Do you have problems with accidental loss of urine?
- How many months or years have you had leakage?
- Do you have to wear pads or protective clothing to prevent or help with urinary loss? If so, how many pads do you wear a day?
- How many trips do you make to the bathroom during the day? At night?
- Do you ever wet the bed while sleeping?
- Are you bothered by a strong sense of urgency to void? Can you overcome it?
- Do you sometimes fail to reach the bathroom in time?
- Does the sound, sight, or feel of running water cause you to lose urine?
- Do you lose urine when you cough, sneeze, run, or lift heavy objects?
- Do you lose urine with posture changes, standing, or walking?
- Do you feel as though you are constantly wet?
- Do you feel as though your bladder is completely empty after passing urine?
- Do you have difficulty starting a stream of urine?
Take a thorough medical history, as well as a surgical history with emphasison previous bladder or gynecologic procedures.
Also review all prescription medications.
48-hour voiding diary. Give the patient a voiding diary to fill out 48 hours prior to her office visit. The reason: The diary often reveals more information than can be elicited from the patient’s history. For example, it may highlight daily activities associated with voiding, such as excessive consumption of liquids, high caffeine intake, high-impact exercise, and so on.
Quality-of-life assessment. An objective means of quantifying the effects of incontinence on the woman’s quality of life is recommended. We use the short form of the Incontinence Impact Questionnaire and the Urinary Distress Inventory.
Step 2Perform ‘eyeball’ cystometry, a simple and revealing office test
Ask the patient to go to the restroom and comfortably empty her bladder into a urine-collection device to determine the amount voided. Have a nurse measure the postvoid residual using a soft red rubber catheter. A sample can be taken for urinalysis and, if necessary, sent for culture.
Next, perform a simple filling or “eyeball” cystometry. Connect a Toomey syringe to the end of the red rubber catheter and pour sterile water into it. Ask the patient to tell you when she feels the first sensation of filling, first desire to void, strong urge to void, and maximum capacity, recording the levels at which each occurs. During filling, any evidence of bladder contraction will be revealed by a rise in the column of water. Record any significant discomfort or other observations during the filling portion of the study. When maximum capacity is reached, remove the catheter.
Step 3Conduct a thorough physical assessment
With the patient in the supine position, separate the labia and ask her to cough forcefully and perform the Valsalva maneuver 3 times, recording any evidence of water or urine loss through the urethral meatus. (If the patient has advanced pelvic organ prolapse, try to reduce the prolapse to eliminate any anatomic distortion of the urethra.)
Then ask the patient to stand with a full bladder and to squat, again having her cough forcefully 3 times. Record any additional urinary loss. Finally, ask the patient to void and again record the amount voided.
After the patient has emptied her bladder, again ask her to cough forcefully 3 times in the supine position, noting any evidence of leakage from the urethra (empty supine stress test).
Perform an overall inspection of the perineum and external genitalia and record a description in the patient’s chart.
Attempt to elicit an anal wink, and perform a brief neurological examination to ensure that spinal cord segments S2, S3, and S4 are intact. Next, gently insert a finger into the vagina and ask the patient to forcefully squeeze around it. Record the forcefulness of the squeeze on a scale of 0 to 5, with 0 being no appreciable movement and 5 being the most forceful squeeze possible. During this portion of the exam, instruct the patient on how to perform a Kegel exercise without recruiting the muscles of the buttocks and the abdominal wall.
Next, use a finger to gently massage the urethra, looking for any possible discharge from the urethral meatus that would be consistent with urethral diverticulum. Also note any tenderness or pain elicited during the exam.
Inserting a half-speculum into the vagina, displace the rectum away from the bladder. As the patient performs a Valsalva maneuver or coughs forcefully, evaluate the support of the anterior vaginal wall. Then turn the speculum 180 degrees and displace the bladder anteriorly, examining the posterior pelvic wall for any signs of prolapse. Also note any urogenital atrophy.
Finally, use a full speculum to evaluate the vaginal apex and cervix (if the patient has not had a hysterectomy). Bimanual and abdominal examinations also are important to rule out any abdominal or pelvic mass that could be irritating or causing pressure on the bladder.
Reexamine the patient for evidence of prolapse when she is standing. Some cases are difficult to detect when the patient is supine.
Step 4Begin multipronged therapy
Bladder retraining. Review the patient’s history, voiding diary, and findings from the physical exam to identify an appropriate treatment. After sharing all findings with the patient, instruct the patient on bladder retraining, which has 3 main components: education, scheduled voiding, and positive reinforcement. (Bladder retraining can be an extremely successful modality.)
Education consists of explaining the pathophysiology of overactive bladder and answering any questions the patient may have, so she understands why she is having the problem.
For scheduled voiding—also known as bladder retraining—examine the voiding diary to determine the approximate length of time between voids in a day. For instance, if the patient voids every hour, we generally ask her to continue doing so for the first week of retraining. The following week, we increase the interval to an hour and 15 minutes, the third week to 1.5 hours, and so on, with an ultimate goal of 3 hours between voids.
We also give instructions on pelvic floor rehabilitation or Kegel exercises. If the patient is able to voluntarily contract her pelvic floor muscles adequately, we recommend an exercise regimen that involves contracting her muscles numerous times a day for at least 10 seconds each time. If she is unable to contract her muscles or has a “dead” pelvic floor, she is referred to a physical therapist for biofeedback and possibly electrical stimulation.
We also instruct the patient to avoid racing to the bathroom when she feels an urge to void. Instead, have her stop, contract her pelvic floor muscles, and allow the urge to pass. She can then walk comfortably to the bathroom.
As mentioned earlier, the voiding diary may highlight problems such as excessive intake of fluids.
Local estrogen therapy can be added if there are signs of urogenital atrophy.
Medical therapy. In addition to education, timed voids, pelvic floor rehabilitation, and estrogen therapy, we usually start the extended-release form of tolterodine (4 mg) or oxybutynin (10 mg), which are antimuscarinic agents. (See “Treating overactive bladder: An expanding ‘pharmacopeia’”).
We ask her to return for a follow-up visit in 4 to 6 weeks to inquire about her symptoms and any significant side effects. We ask the patient to prepare another voiding diary for that visit so we can objectively measure decreases in frequency, urgency, and urge incontinence.
Dose adjustment is very important in patients with overactive bladder. If the woman is experiencing minimal effect with no side effects, titrate the dosage upward. If she is having good effect with significant side effects, decrease the dosage or consider switching to the transdermal form of oxybutynin.
When all these conditions are met and the patient remains severely affected, we perform multichannel urodynamic testing along with cystoscopy. If the testing supports the diagnosis of overactive bladder, and all other mechanisms for improvement have been exhausted, we counsel the patient about intravesical administration of botulism toxin, which relaxes skeletal and smooth muscle by preventing release of acetocholine from nerve terminal endings. Another option is sacroneuromodulation (InterStim therapy).
Antimuscarinic medications have been the mainstay of pharmacologic therapy for overactive bladder. In fact, the 2 most commonly prescribed drugs for overactive bladder are antimuscarinics: oxybutynin and tolterodine. Other medications also are available that affect different aspects of the neurophysiology of micturition.
Oxybutynin. The bladder contains 5 subtypes of muscarinic receptors, with a predominance of M2 and M3 subtypes. Oxybutynin has some selectivity for M3 receptors. It has been shown to inhibit bladder contractions, but because of muscarinic blockade in other parts of the body, it can have bothersome side effects. For example, oxybutynin has shown a higher affinity for muscarinic receptors in the parotid gland than the bladder, leading to dry mouth. In addition, the active metabolite of oxybutynin, N-Desethyl-oxybutynin, can reach concentrations that are 6 times the parent compound after oral administration.9 Other side effects include constipation, gastrointestinal upset, and blurry vision.
Alternate routes of administration have been developed to improve tolerability, including extended-release oral formulations, rectal administration, and a transdermal patch. Oxybutynin XL is the extended-release form, which incorporates a push-pull osmotic system. In parallel randomized, controlled clinical trials comparing immediate- and extended-release oxybutynin, oxybutynin XL was just as effective as the immediate-release formulation but had fewer side effects.10-12 Absorption of oxybutynin XL may occur to a larger degree in the colon than in the stomach and proximal small intestine, thereby decreasing conversion to N-Desethyl-oxybutynin. Oxybutynin XL comes in 3 doses (5, 10, and 15 mg) and offers the advantage of dose titration, a very important aspect of management.
A transdermal version of oxybutynin was recently released. In theory, transdermal treatment offers potential advantages such as more stable plasma concentrations and lower presystemic metabolism, which may decrease the primary active metabolite of N-Desethyl-oxybutynin. In a randomized, placebo-controlled trial of 3 doses of transdermal oxybutynin (1.3, 2.6, and 3.9 mg daily), only the highest dose out-performed placebo for median changes in incontinence episodes, frequency, and normal void volume.13 Quality of life also was significantly improved with the 3.9-mg dose. Anticholinergic side effects were comparable between active and placebo groups.
Tolterodine is a potent muscarinic-receptor antagonist without selectivity for particular subtypes. It is marketed in both immediate- and extended-release (tolterodine LA) formulations. A recent series compared both forms of the drug with placebo in 1,529 adults with overactive bladder.14 The primary measure of efficacy was change in the mean number of incontinent episodes weekly. Both medications showed a significantly better response than placebo. Dry mouth was significantly lower with tolterodine LA. When the authors used median values instead of mean values (because of non-normal distributed data), tolterodine LA showed improved efficacy over the immediate-release formulation. This study was sponsored by the drug’s manufacturer.
Comparisons of oxybutynin and tolterodine. In a prospective, double-blind, head-to-head study of the 2 drugs sponsored by the manufacturer of oxybutynin XL, 378 men and women were randomized to receive oxybutynin XL (10 mg daily) or tolterodine immediate-release (2 mg twice daily).15 Inclusion criteria included 7 or more episodes of urge incontinence per week and at least 10 voids per 24 hours. The Overactive Bladder: Judging Effective Control and Treatment (OBJECT) trial demonstrated greater efficacy with extended-release oxybutynin than with tolterodine, although both medications decreased weekly episodes of urge incontinence. Oxybutynin XL also showed a small but significant advantage for mixed incontinence and urinary frequency. Dry mouth and central nervous system side effects were similar for the 2 drugs.
A second head-to-head study also was sponsored by the manufacturer of oxybutynin—this one a randomized, double-blind comparison of the extended-release versions of both drugs. The Overactive Bladder: Performance of Extended Release Agents (OPERA) trial randomized 790 women to oxybutynin XL (10 mg daily) or tolterodine LA (4 mg daily).16 Inclusion criteria included at least 21 to 60 incontinent episodes per week and urgency and frequency exceeding 10 episodes per 24 hours. The drugs were similar in both measures, as well as in side effects, although dry mouth, usually mild, was more common among oxybutynin users. However, oxybutynin was significantly more effective in reducing micturition frequency, and 23% of women taking oxybutynin reported no episodes of incontinence, compared with 16.8% of women taking tolterodine. This finding was significant.
A third head-to-head study involved 2 separate trials conducted in parallel, with individual centers evaluating only 1 drug. In it, 1,289 patients were randomized to open-label treatment with either 2 mg or 4 mg of tolterodine LA in 1 trial and with 5 mg or 10 mg of oxybutynin XL in the other.17Inclusion criteria included 18 years of age or greater and symptoms of urinary urgency and frequency. After 8 weeks, tolterodine LA (4 mg) was found to be significantly more effective than either dose of oxybutynin XL, while tolterodine LA (2 mg) was similar to both doses of oxybutynin XL. This study was hampered by the fact that no data were supplied on the number of patients with isolated frequency and urgency versus those who had urge incontinence. Also, no objective parameters such as a voiding diary were utilized.
Three antimuscarinic drugs in the pipeline. Other antimuscarinic agents include trospium, a quaternary amine with some antimuscarinic activity. Because of its structure, it does not cross the brain barrier well and may thus have fewer central side effects.
Two new drugs awaiting approval from the US Food and Drug Administration are darifenacin, which has high selectivity for M3 receptors, and solifenacin, an M3 antagonist with greater selectivity for the bladder than the salivary glands in animal models.
All 3 drugs should be available in the United States some time in 2004.
Antiadrenergic medications. Because the bladder also contains alpha- and beta-adrenergic receptors, antiadrenergic medications have been developed. Currently available alpha-adrenergic agonists include ephedrine, phenyl-propanolamine, and pseudoephedrine. Beta-adrenergic agonists include isoproterenol and terbutaline.
Serotonergic agents. Both sympathetic and parasympathetic autonomic nuclei—as well as urethral sphincter motor nuclei—receive serotonergic input from the Raphe nuclei in the caudal brain stem. Serotonergic pathways generally enhance urine storage by activating sympathetic pathways and inhibiting parasympathetic pathways. Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor that may be effective against both urge and stress incontinence. It is currently awaiting approval by the US Food and Drug Administration.
Implications of the placebo effect
We lack a complete understanding of how all the parts of this process interact and why. Most of the medications in use affect only 1 part of the complex process that governs urine storage and elimination.
In controlled trials, patients on placebo have experienced 30% improvement—or greater—in their symptoms. This would seem to suggest that education, behavioral retraining, and attention from physicians are responsible for some of the improvement. Indeed, in a recent systematic review of 32 trials involving 6,800 participants, Herbison et al8 found significant but relatively small differences between anticholinergic medications and placebo for many of the outcomes studied. Obviously, we have more to learn about the intricacies of this condition before we can eliminate it completely.
1. Stewart W, Herzog R, Wein A, et al. Prevalence of overactive bladder in the US: results from the Noble Program. Poster presented at the Second International Consultation on Incontinence, July 2001, Paris, France.
2. Voelker R. International group seeks to dispel incontinence ‘taboo.’ JAMA. 1998;280:951.-
3. Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurol Urodynamics. 2002;21:167-178.
4. Walters MD, Diaz K. Q-tip test: a study of continent and incontinent women. Obstet Gynecol. 1987;70:208-211.
5. Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355.-
6. Wagner TH, Hu TW. Economic costs of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:127.-
7. Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204.-
8. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
9. Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate release oxybutynin. J Clin Pharmacol. 1999;39:289.-
10. Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999;161:1809.-
11. Birns J, Lukkari E, Malone-Lee JG. A randomized controlled trial comparing the efficacy of controlled-release oxybutynin tablets (10 mg once daily) with conventional oxybutynin tablets (5 mg twice daily) in patients whose symptoms were stabilized on 5-mg twice daily of oxybutynin. BJU Int. 2000;85:793.-
12. Versi E, Appell R, Mobley D, Patton W, Saltzstein D. Dry mouth with conventional and controlled released oxybutynin in urinary incontinence. The Ditropan XL Study Group. Obstet Gynecol. 2000;95:718.-
13. Dmochowski RR, Davila GW, Zinner NR, et al. Efficacy and safety of transdermal oxybutynin in patients with urge and mixed urinary incontinence. J Urol. 2002;168:580-586.
14. Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57:414.-
15. Appell RA, Sand P, Dmochowski R, et al. for the OBJECT Study Group Prospective randomized controlled trial of extended release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: results of the OBJECT Study. Mayo Clin Proc. 2001;76:358-363.
16. Diokno AC, Appell RA, Sand PK, et al. for the OPERA Study Group Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
17. Sussman D, Garely A. Treatment of overactive bladder with once-daily extended-release tolterodine or oxybutynin: the Antimuscarinic Clinical Effectiveness Trial (ACET). Curr Hosp Res Opin. 2002;18:177-184.
1. Stewart W, Herzog R, Wein A, et al. Prevalence of overactive bladder in the US: results from the Noble Program. Poster presented at the Second International Consultation on Incontinence, July 2001, Paris, France.
2. Voelker R. International group seeks to dispel incontinence ‘taboo.’ JAMA. 1998;280:951.-
3. Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurol Urodynamics. 2002;21:167-178.
4. Walters MD, Diaz K. Q-tip test: a study of continent and incontinent women. Obstet Gynecol. 1987;70:208-211.
5. Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355.-
6. Wagner TH, Hu TW. Economic costs of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:127.-
7. Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162:204.-
8. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
9. Gupta SK, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate release oxybutynin. J Clin Pharmacol. 1999;39:289.-
10. Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999;161:1809.-
11. Birns J, Lukkari E, Malone-Lee JG. A randomized controlled trial comparing the efficacy of controlled-release oxybutynin tablets (10 mg once daily) with conventional oxybutynin tablets (5 mg twice daily) in patients whose symptoms were stabilized on 5-mg twice daily of oxybutynin. BJU Int. 2000;85:793.-
12. Versi E, Appell R, Mobley D, Patton W, Saltzstein D. Dry mouth with conventional and controlled released oxybutynin in urinary incontinence. The Ditropan XL Study Group. Obstet Gynecol. 2000;95:718.-
13. Dmochowski RR, Davila GW, Zinner NR, et al. Efficacy and safety of transdermal oxybutynin in patients with urge and mixed urinary incontinence. J Urol. 2002;168:580-586.
14. Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57:414.-
15. Appell RA, Sand P, Dmochowski R, et al. for the OBJECT Study Group Prospective randomized controlled trial of extended release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: results of the OBJECT Study. Mayo Clin Proc. 2001;76:358-363.
16. Diokno AC, Appell RA, Sand PK, et al. for the OPERA Study Group Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687-695.
17. Sussman D, Garely A. Treatment of overactive bladder with once-daily extended-release tolterodine or oxybutynin: the Antimuscarinic Clinical Effectiveness Trial (ACET). Curr Hosp Res Opin. 2002;18:177-184.



