User login
2015 Update on obstetrics
Over the past year, much attention has been devoted to labor curves. Is the original Friedman labor curve, which dates to the 1950s, still applicable today? Or do contemporary women labor differently? And if we update our approach to labor management, can we reduce the rate of primary cesarean?
In this Update, we explore these questions, as well as two others:
- How do we minimize infectious morbidity in pregnancy?
- How much prenatal screening is too much?
Is adherence to new labor curves the best way to reduce the rate of primary cesarean?
American College of Obstetricians and Gynecologists. Obstetric Care Consensus No. 1: Safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
Cohen WR, Friedman EA. Perils of the new labor management guidelines [published online ahead of print September 16, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.09.008.
In 2012, the cesarean delivery rate in the United States remained at 32.8%, a high percentage when one considers the increased risks that major abdominal surgery poses in both the short and long term (blood loss, transfusion, infection, venous thromboembolism, abnormal placentation, hysterectomy).1 The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) have made it a priority to reduce the cesarean delivery rate, focusing their efforts on the primary cesarean. In March 2014, they jointly issued guidelines on the “Safe prevention of the primary cesarean delivery,” highlighting labor dystocia as a top cause.
When contemporary data from the Consortium on Safe Labor were applied to the original Friedman labor curve, investigators found that the active phase of labor may be slower than previously thought.2 The maximum slope for the rate of cervical change was not observed until 6 cm of dilation. This finding potentially changes the point at which arrest of the active phase may be declared. The maximum duration of augmentation with oxytocin also has been extended, based on studies that demonstrated increased vaginal delivery rates.
The Consortium on Safe Labor proposed that, by subjecting a contemporary population to decades-old standards, we have been intervening with primary cesarean too early in the treatment of labor dystocia.
What the guidelines say
The new recommendations from ACOG-SMFM suggest that arrest of the active phase of labor can be declared only when the patient is dilated at least 6 cm with ruptured membranes after either 4 hours of adequate uterine contractions or at least 6 hours of oxytocin administration with inadequate uterine contractions or no cervical change.
Although the recommendations state that there is no maximum duration of the second stage of labor, we may increase the vaginal delivery rate by increasing the duration of pushing to 2 hours for a multiparous patient and 3 hours for a nulliparous patient (with an additional hour when an epidural is given).
Are the recommendations ready for prime time?
In response to the recommendations, Cohen and Friedman (author of the original labor curve) published “Perils of the new labor management guidelines,” cited above. In this commentary, they caution against universal acceptance of the guidelines without further validation. They argue that the analytical method used—and not labor itself—has changed, with possible selection biases and unadjusted confounders altering the shape of the dilatation curve. Cohen and Friedman suggest that serial evaluation of the patient is preferable to an arbitrary cutoff of 6 cm.
They also criticize other aspects of the guidelines, focusing on universal use of intrauterine pressure catheters, amniotomy, and a specific duration of pushing without consideration of descent. A “one size fits all” approach may incur risk to both the mother and the fetus without proven benefit, they contend. Clinical judgment and continuous evaluation of the likelihood and safety of vaginal delivery also are encouraged rather than a reliance on labor curves in isolation.
They urge further validation before adoption of the recommendations. “If we direct our clinical and basic science investigations to the goal of practicing obstetrics in a manner that optimizes maternal and newborn outcomes, the ideal cesarean delivery rate, whatever it may be, will follow,” they write.
What this EVIDENCE means for practice
Proceed with caution when applying labor curves to patients. Use clinical judgment in conjunction with any new guidelines.
Be vigilant for infectious threats to your obstetric population
Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014;124(5):1005–1010.
American College of Obstetricians and Gynecologists. Committee Opinion No. 614: Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124(6):1241–1244.
American College of Obstetricians and Gynecologists. Committee Opinion No. 608: Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124(3):648–651.
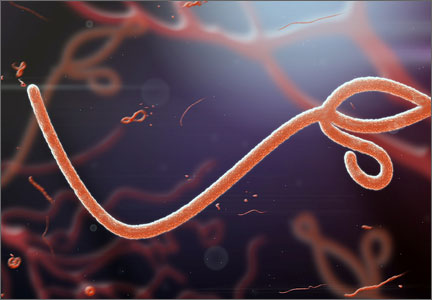
We no longer consider pregnancy an immunosuppressed state but, rather, a more immune-modulated system. However, there is no question that the unique physiologic state of pregnancy places a woman and her fetus at increased risk for infection. This was devastatingly obvious during the H1N1 epidemic of 2009 and was reemphasized during a 2014 outbreak of Listeria monocytogenes. We are reminded again during the largest Ebola virus outbreak in history in West Africa, where women have been disproportionately affected.
No neonates have survived Ebola
Although Ebola infections in the United States have been very few, vigilance for people at risk of infection and preparedness to act in the case of infection are vitally important.
The Ebola virus is thought to be spread to humans through contact with infected fruit bats or primates. Human-to-human transmission occurs through direct contact with blood or body fluids (urine, feces, sweat, saliva, breast milk, vomit, semen) of an infected person or contaminated objects (needles, syringes). The incubation period is 2 to 21 days (average, 8–10 days).
Infected people become contagious only upon the appearance of fever and symptoms, which include headache, muscle pain, fatigue, weakness, diarrhea, abdominal pain, vomiting, bleeding, and bruising. The differential diagnosis includes malaria, typhoid, Lassa fever, meningococcal disease, influenza, and Marburg virus.
Treatment of Ebola is supportive care and isolation (standard, contact, and droplet precautions). Prevention is through infection-control precautions and isolation and testing of those exposed, with monitoring for 21 days.
Although pregnant women are not thought to be more susceptible to infection, they are at increased risk of severe illness and mortality, as well as spontaneous abortion and pregnancy-related hemorrhage. No neonates of women infected with Ebola have survived to date.
The CDC recommends that physicians screen patients who have traveled to West Africa and those with fevers and implement appropriate isolation and infection-control precautions. Many hospitals have developed Ebola task forces with this in mind.
Updated information is available at www.cdc.gov/vhf/ebola/index.html.
Pregnant women are highly susceptible to Listeriosis
A nationwide food recall in mid-2014 prompted significant media attention to L monocytogenes, particularly its effect on pregnant women, who have an incidence of Listerial infection 13 times higher than the general population. Although maternal illness is relatively mild, ranging from a complete lack of symptoms to febrile diarrhea, there is an increased risk to the fetus or neonate of loss, preterm labor, neonatal sepsis, meningitis, and death. The perinatal mortality rate is 29%.
The mainstay of prevention during pregnancy is improved food safety and handling, as well as counseling of pregnant women to avoid unpasteurized soft cheeses, raw milk, and unwashed fruits and vegetables, and to avoid or heat thoroughly lunch meats and hot dogs.
When a pregnant woman is exposed to Listeria, management depends on the clinical scenario, as outlined by ACOG:
- Asymptomatic pregnant women do not require testing, treatment, or fetal surveillance. Any development of symptoms within 2 months may justify further evaluation, however.
- Pregnant women with mild gastro-intestinal or flulike symptoms but no fever also can be managed expectantly. Blood cultures may be appropriate; if positive, antibiotic therapy should be initiated.
- A febrile pregnant woman should have blood cultures assessed and be started on antibiotics. The preferred regimen is intravenous ampicillin 6 g/day with or without gentamicin for 14 days. If delivery occurs, placental cultures may be assessed. Listeriosis also can be diagnosed by amniocentesis. Stool cultures are not recommended.
Influenza is largely preventable
It is important to remember that one of the most dangerous viruses for pregnant women can be prevented. However, only 38% to 52% of women who should have received the influenza vaccine around the time of pregnancy actually did so between 2009 and 2013, according to the ACOG Committee Opinion cited above. Pregnant and postpartum women are at increased risk of serious illness, prolonged hospitalization, and death from influenza infection.
The vaccine is safe and effective. Not only does it prevent maternal morbidity and mortality, but it reduces neonatal complications. Inactivated vaccine is recommended for all pregnant women at any gestational age during the flu season.
Because many women are hesitant to accept the vaccine, accurate education is essential to dispel misconceptions about it and its components. It has been shown that if an obstetric clinician recommends the vaccine and makes it available, pregnant patients are five to 50 times more likely to receive it. As obstetricians, we are compelled to make this a priority in our practice.
What this EVIDENCE means for practice
Be alert and ready to act if an infectious threat is noted in your obstetric population. Get your flu shot. Give it to your obstetric patients. And don’t forget that ACOG also supports the administration of one dose of the tetanus, diphtheria, and pertussis vaccine during each pregnancy.
How much prenatal screening is too much?
Goetzinger KR, Odibo AO. Screening for abnormal placentation and adverse pregnancy outcomes with maternal serum biomarkers in the second trimester. Prenatal Diagn. 2014;34(7):635–641.
D’Antonio F, Rijo C, Thilaganathan B, et al. Association between first-trimester maternal serum pregnancy associated plasma protein-A and obstetric complications. Prenatal Diagn. 2013;33(9):839–847.
Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
Martin A, Krishna I, Martina B, et al. Can the quantity of cell-free fetal DNA predict preeclampsia: a systematic review. Prenatal Diagn. 2014;34(7): 685–691.
Audibert F, Boucoiran I, An N, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383.e1–e8.
Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242.
The placenta of a normal pregnancy secretes small amounts of a variety of biomarkers such as alpha-fetoprotein (AFP), human chorionic gonadotropin, unconjugated estriol, inhibin A, pregnancy-associated placental protein A (PAPP-A), soluble fms-like tyrosine kinase, and placental growth factor.
The association between abnormal maternal serum biomarkers and abnormal pregnancy outcomes has been known since the 1970s, when elevated AFP was noted in pregnancies with fetal open neural tube defects. Shortly thereafter, low levels of AFP were associated with fetuses with trisomy 21.
One theory is that the abnormality in pregnancy leads to abnormal regulation at the level of the fetal-placental interface and over- or under-secretion of the various biomarkers. An offshoot of this theory is the idea that abnormal placentation (ie, preeclampsia, fetal growth restriction, accreta) also may be reflected in elevated or suppressed secretion of placental biomarkers, which could be used to screen for these conditions during pregnancy.
PAPP-A is a placental serum marker that is a component of first-trimester genetic screening. It is a marker of placental function, and low levels have been associated with fetal growth restriction, preterm birth, preeclampsia, and fetal loss. Another first-trimester marker associated with adverse outcomes is cell-free fetal DNA. This DNA, found in the maternal blood, is a product of placental apoptosis, and elevated levels have been demonstrated in women who develop preeclampsia.
Although many of the biomarkers listed here are not available specifically as a clinical screening test in the United States, the link to common genetic screens makes it tempting to try to add prediction of preeclampsia and other information to an existing test. If specific numbers are reported on the genetic screen for the different markers, that information is already there, and some companies may flag abnormally high or low levels.
However, although the association between abnormal pregnancy outcomes and abnormal biomarkers is well established in the literature, the clinical predictive value is not—nor is there always an effective intervention available. One could argue that low-dose aspirin, which is already recommended for patients with a prior delivery before 34 weeks due to preeclampsia, or more than one prior pregnancy with preeclampsia, could be recommended for patients identified on early screens to be at increased risk for preeclampsia. This approach should be tested in randomized clinical trials before universal adoption.
What this EVIDENCE means for practice
Although it is tempting to use associations to predict adverse events, the clinical value of doing so has not yet been proven. Exercise caution before potentially causing concern for both you and your patient.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):1–67.
2. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Consortium on Safe Labor. Obstet Gynecol. 2010;116(6):1281–1287.
Over the past year, much attention has been devoted to labor curves. Is the original Friedman labor curve, which dates to the 1950s, still applicable today? Or do contemporary women labor differently? And if we update our approach to labor management, can we reduce the rate of primary cesarean?
In this Update, we explore these questions, as well as two others:
- How do we minimize infectious morbidity in pregnancy?
- How much prenatal screening is too much?
Is adherence to new labor curves the best way to reduce the rate of primary cesarean?
American College of Obstetricians and Gynecologists. Obstetric Care Consensus No. 1: Safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
Cohen WR, Friedman EA. Perils of the new labor management guidelines [published online ahead of print September 16, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.09.008.
In 2012, the cesarean delivery rate in the United States remained at 32.8%, a high percentage when one considers the increased risks that major abdominal surgery poses in both the short and long term (blood loss, transfusion, infection, venous thromboembolism, abnormal placentation, hysterectomy).1 The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) have made it a priority to reduce the cesarean delivery rate, focusing their efforts on the primary cesarean. In March 2014, they jointly issued guidelines on the “Safe prevention of the primary cesarean delivery,” highlighting labor dystocia as a top cause.
When contemporary data from the Consortium on Safe Labor were applied to the original Friedman labor curve, investigators found that the active phase of labor may be slower than previously thought.2 The maximum slope for the rate of cervical change was not observed until 6 cm of dilation. This finding potentially changes the point at which arrest of the active phase may be declared. The maximum duration of augmentation with oxytocin also has been extended, based on studies that demonstrated increased vaginal delivery rates.
The Consortium on Safe Labor proposed that, by subjecting a contemporary population to decades-old standards, we have been intervening with primary cesarean too early in the treatment of labor dystocia.
What the guidelines say
The new recommendations from ACOG-SMFM suggest that arrest of the active phase of labor can be declared only when the patient is dilated at least 6 cm with ruptured membranes after either 4 hours of adequate uterine contractions or at least 6 hours of oxytocin administration with inadequate uterine contractions or no cervical change.
Although the recommendations state that there is no maximum duration of the second stage of labor, we may increase the vaginal delivery rate by increasing the duration of pushing to 2 hours for a multiparous patient and 3 hours for a nulliparous patient (with an additional hour when an epidural is given).
Are the recommendations ready for prime time?
In response to the recommendations, Cohen and Friedman (author of the original labor curve) published “Perils of the new labor management guidelines,” cited above. In this commentary, they caution against universal acceptance of the guidelines without further validation. They argue that the analytical method used—and not labor itself—has changed, with possible selection biases and unadjusted confounders altering the shape of the dilatation curve. Cohen and Friedman suggest that serial evaluation of the patient is preferable to an arbitrary cutoff of 6 cm.
They also criticize other aspects of the guidelines, focusing on universal use of intrauterine pressure catheters, amniotomy, and a specific duration of pushing without consideration of descent. A “one size fits all” approach may incur risk to both the mother and the fetus without proven benefit, they contend. Clinical judgment and continuous evaluation of the likelihood and safety of vaginal delivery also are encouraged rather than a reliance on labor curves in isolation.
They urge further validation before adoption of the recommendations. “If we direct our clinical and basic science investigations to the goal of practicing obstetrics in a manner that optimizes maternal and newborn outcomes, the ideal cesarean delivery rate, whatever it may be, will follow,” they write.
What this EVIDENCE means for practice
Proceed with caution when applying labor curves to patients. Use clinical judgment in conjunction with any new guidelines.
Be vigilant for infectious threats to your obstetric population
Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014;124(5):1005–1010.
American College of Obstetricians and Gynecologists. Committee Opinion No. 614: Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124(6):1241–1244.
American College of Obstetricians and Gynecologists. Committee Opinion No. 608: Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124(3):648–651.
We no longer consider pregnancy an immunosuppressed state but, rather, a more immune-modulated system. However, there is no question that the unique physiologic state of pregnancy places a woman and her fetus at increased risk for infection. This was devastatingly obvious during the H1N1 epidemic of 2009 and was reemphasized during a 2014 outbreak of Listeria monocytogenes. We are reminded again during the largest Ebola virus outbreak in history in West Africa, where women have been disproportionately affected.
No neonates have survived Ebola
Although Ebola infections in the United States have been very few, vigilance for people at risk of infection and preparedness to act in the case of infection are vitally important.
The Ebola virus is thought to be spread to humans through contact with infected fruit bats or primates. Human-to-human transmission occurs through direct contact with blood or body fluids (urine, feces, sweat, saliva, breast milk, vomit, semen) of an infected person or contaminated objects (needles, syringes). The incubation period is 2 to 21 days (average, 8–10 days).
Infected people become contagious only upon the appearance of fever and symptoms, which include headache, muscle pain, fatigue, weakness, diarrhea, abdominal pain, vomiting, bleeding, and bruising. The differential diagnosis includes malaria, typhoid, Lassa fever, meningococcal disease, influenza, and Marburg virus.
Treatment of Ebola is supportive care and isolation (standard, contact, and droplet precautions). Prevention is through infection-control precautions and isolation and testing of those exposed, with monitoring for 21 days.
Although pregnant women are not thought to be more susceptible to infection, they are at increased risk of severe illness and mortality, as well as spontaneous abortion and pregnancy-related hemorrhage. No neonates of women infected with Ebola have survived to date.
The CDC recommends that physicians screen patients who have traveled to West Africa and those with fevers and implement appropriate isolation and infection-control precautions. Many hospitals have developed Ebola task forces with this in mind.
Updated information is available at www.cdc.gov/vhf/ebola/index.html.
Pregnant women are highly susceptible to Listeriosis
A nationwide food recall in mid-2014 prompted significant media attention to L monocytogenes, particularly its effect on pregnant women, who have an incidence of Listerial infection 13 times higher than the general population. Although maternal illness is relatively mild, ranging from a complete lack of symptoms to febrile diarrhea, there is an increased risk to the fetus or neonate of loss, preterm labor, neonatal sepsis, meningitis, and death. The perinatal mortality rate is 29%.
The mainstay of prevention during pregnancy is improved food safety and handling, as well as counseling of pregnant women to avoid unpasteurized soft cheeses, raw milk, and unwashed fruits and vegetables, and to avoid or heat thoroughly lunch meats and hot dogs.
When a pregnant woman is exposed to Listeria, management depends on the clinical scenario, as outlined by ACOG:
- Asymptomatic pregnant women do not require testing, treatment, or fetal surveillance. Any development of symptoms within 2 months may justify further evaluation, however.
- Pregnant women with mild gastro-intestinal or flulike symptoms but no fever also can be managed expectantly. Blood cultures may be appropriate; if positive, antibiotic therapy should be initiated.
- A febrile pregnant woman should have blood cultures assessed and be started on antibiotics. The preferred regimen is intravenous ampicillin 6 g/day with or without gentamicin for 14 days. If delivery occurs, placental cultures may be assessed. Listeriosis also can be diagnosed by amniocentesis. Stool cultures are not recommended.
Influenza is largely preventable
It is important to remember that one of the most dangerous viruses for pregnant women can be prevented. However, only 38% to 52% of women who should have received the influenza vaccine around the time of pregnancy actually did so between 2009 and 2013, according to the ACOG Committee Opinion cited above. Pregnant and postpartum women are at increased risk of serious illness, prolonged hospitalization, and death from influenza infection.
The vaccine is safe and effective. Not only does it prevent maternal morbidity and mortality, but it reduces neonatal complications. Inactivated vaccine is recommended for all pregnant women at any gestational age during the flu season.
Because many women are hesitant to accept the vaccine, accurate education is essential to dispel misconceptions about it and its components. It has been shown that if an obstetric clinician recommends the vaccine and makes it available, pregnant patients are five to 50 times more likely to receive it. As obstetricians, we are compelled to make this a priority in our practice.
What this EVIDENCE means for practice
Be alert and ready to act if an infectious threat is noted in your obstetric population. Get your flu shot. Give it to your obstetric patients. And don’t forget that ACOG also supports the administration of one dose of the tetanus, diphtheria, and pertussis vaccine during each pregnancy.
How much prenatal screening is too much?
Goetzinger KR, Odibo AO. Screening for abnormal placentation and adverse pregnancy outcomes with maternal serum biomarkers in the second trimester. Prenatal Diagn. 2014;34(7):635–641.
D’Antonio F, Rijo C, Thilaganathan B, et al. Association between first-trimester maternal serum pregnancy associated plasma protein-A and obstetric complications. Prenatal Diagn. 2013;33(9):839–847.
Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
Martin A, Krishna I, Martina B, et al. Can the quantity of cell-free fetal DNA predict preeclampsia: a systematic review. Prenatal Diagn. 2014;34(7): 685–691.
Audibert F, Boucoiran I, An N, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383.e1–e8.
Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242.
The placenta of a normal pregnancy secretes small amounts of a variety of biomarkers such as alpha-fetoprotein (AFP), human chorionic gonadotropin, unconjugated estriol, inhibin A, pregnancy-associated placental protein A (PAPP-A), soluble fms-like tyrosine kinase, and placental growth factor.
The association between abnormal maternal serum biomarkers and abnormal pregnancy outcomes has been known since the 1970s, when elevated AFP was noted in pregnancies with fetal open neural tube defects. Shortly thereafter, low levels of AFP were associated with fetuses with trisomy 21.
One theory is that the abnormality in pregnancy leads to abnormal regulation at the level of the fetal-placental interface and over- or under-secretion of the various biomarkers. An offshoot of this theory is the idea that abnormal placentation (ie, preeclampsia, fetal growth restriction, accreta) also may be reflected in elevated or suppressed secretion of placental biomarkers, which could be used to screen for these conditions during pregnancy.
PAPP-A is a placental serum marker that is a component of first-trimester genetic screening. It is a marker of placental function, and low levels have been associated with fetal growth restriction, preterm birth, preeclampsia, and fetal loss. Another first-trimester marker associated with adverse outcomes is cell-free fetal DNA. This DNA, found in the maternal blood, is a product of placental apoptosis, and elevated levels have been demonstrated in women who develop preeclampsia.
Although many of the biomarkers listed here are not available specifically as a clinical screening test in the United States, the link to common genetic screens makes it tempting to try to add prediction of preeclampsia and other information to an existing test. If specific numbers are reported on the genetic screen for the different markers, that information is already there, and some companies may flag abnormally high or low levels.
However, although the association between abnormal pregnancy outcomes and abnormal biomarkers is well established in the literature, the clinical predictive value is not—nor is there always an effective intervention available. One could argue that low-dose aspirin, which is already recommended for patients with a prior delivery before 34 weeks due to preeclampsia, or more than one prior pregnancy with preeclampsia, could be recommended for patients identified on early screens to be at increased risk for preeclampsia. This approach should be tested in randomized clinical trials before universal adoption.
What this EVIDENCE means for practice
Although it is tempting to use associations to predict adverse events, the clinical value of doing so has not yet been proven. Exercise caution before potentially causing concern for both you and your patient.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Over the past year, much attention has been devoted to labor curves. Is the original Friedman labor curve, which dates to the 1950s, still applicable today? Or do contemporary women labor differently? And if we update our approach to labor management, can we reduce the rate of primary cesarean?
In this Update, we explore these questions, as well as two others:
- How do we minimize infectious morbidity in pregnancy?
- How much prenatal screening is too much?
Is adherence to new labor curves the best way to reduce the rate of primary cesarean?
American College of Obstetricians and Gynecologists. Obstetric Care Consensus No. 1: Safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
Cohen WR, Friedman EA. Perils of the new labor management guidelines [published online ahead of print September 16, 2014]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.09.008.
In 2012, the cesarean delivery rate in the United States remained at 32.8%, a high percentage when one considers the increased risks that major abdominal surgery poses in both the short and long term (blood loss, transfusion, infection, venous thromboembolism, abnormal placentation, hysterectomy).1 The American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) have made it a priority to reduce the cesarean delivery rate, focusing their efforts on the primary cesarean. In March 2014, they jointly issued guidelines on the “Safe prevention of the primary cesarean delivery,” highlighting labor dystocia as a top cause.
When contemporary data from the Consortium on Safe Labor were applied to the original Friedman labor curve, investigators found that the active phase of labor may be slower than previously thought.2 The maximum slope for the rate of cervical change was not observed until 6 cm of dilation. This finding potentially changes the point at which arrest of the active phase may be declared. The maximum duration of augmentation with oxytocin also has been extended, based on studies that demonstrated increased vaginal delivery rates.
The Consortium on Safe Labor proposed that, by subjecting a contemporary population to decades-old standards, we have been intervening with primary cesarean too early in the treatment of labor dystocia.
What the guidelines say
The new recommendations from ACOG-SMFM suggest that arrest of the active phase of labor can be declared only when the patient is dilated at least 6 cm with ruptured membranes after either 4 hours of adequate uterine contractions or at least 6 hours of oxytocin administration with inadequate uterine contractions or no cervical change.
Although the recommendations state that there is no maximum duration of the second stage of labor, we may increase the vaginal delivery rate by increasing the duration of pushing to 2 hours for a multiparous patient and 3 hours for a nulliparous patient (with an additional hour when an epidural is given).
Are the recommendations ready for prime time?
In response to the recommendations, Cohen and Friedman (author of the original labor curve) published “Perils of the new labor management guidelines,” cited above. In this commentary, they caution against universal acceptance of the guidelines without further validation. They argue that the analytical method used—and not labor itself—has changed, with possible selection biases and unadjusted confounders altering the shape of the dilatation curve. Cohen and Friedman suggest that serial evaluation of the patient is preferable to an arbitrary cutoff of 6 cm.
They also criticize other aspects of the guidelines, focusing on universal use of intrauterine pressure catheters, amniotomy, and a specific duration of pushing without consideration of descent. A “one size fits all” approach may incur risk to both the mother and the fetus without proven benefit, they contend. Clinical judgment and continuous evaluation of the likelihood and safety of vaginal delivery also are encouraged rather than a reliance on labor curves in isolation.
They urge further validation before adoption of the recommendations. “If we direct our clinical and basic science investigations to the goal of practicing obstetrics in a manner that optimizes maternal and newborn outcomes, the ideal cesarean delivery rate, whatever it may be, will follow,” they write.
What this EVIDENCE means for practice
Proceed with caution when applying labor curves to patients. Use clinical judgment in conjunction with any new guidelines.
Be vigilant for infectious threats to your obstetric population
Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014;124(5):1005–1010.
American College of Obstetricians and Gynecologists. Committee Opinion No. 614: Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124(6):1241–1244.
American College of Obstetricians and Gynecologists. Committee Opinion No. 608: Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124(3):648–651.
We no longer consider pregnancy an immunosuppressed state but, rather, a more immune-modulated system. However, there is no question that the unique physiologic state of pregnancy places a woman and her fetus at increased risk for infection. This was devastatingly obvious during the H1N1 epidemic of 2009 and was reemphasized during a 2014 outbreak of Listeria monocytogenes. We are reminded again during the largest Ebola virus outbreak in history in West Africa, where women have been disproportionately affected.
No neonates have survived Ebola
Although Ebola infections in the United States have been very few, vigilance for people at risk of infection and preparedness to act in the case of infection are vitally important.
The Ebola virus is thought to be spread to humans through contact with infected fruit bats or primates. Human-to-human transmission occurs through direct contact with blood or body fluids (urine, feces, sweat, saliva, breast milk, vomit, semen) of an infected person or contaminated objects (needles, syringes). The incubation period is 2 to 21 days (average, 8–10 days).
Infected people become contagious only upon the appearance of fever and symptoms, which include headache, muscle pain, fatigue, weakness, diarrhea, abdominal pain, vomiting, bleeding, and bruising. The differential diagnosis includes malaria, typhoid, Lassa fever, meningococcal disease, influenza, and Marburg virus.
Treatment of Ebola is supportive care and isolation (standard, contact, and droplet precautions). Prevention is through infection-control precautions and isolation and testing of those exposed, with monitoring for 21 days.
Although pregnant women are not thought to be more susceptible to infection, they are at increased risk of severe illness and mortality, as well as spontaneous abortion and pregnancy-related hemorrhage. No neonates of women infected with Ebola have survived to date.
The CDC recommends that physicians screen patients who have traveled to West Africa and those with fevers and implement appropriate isolation and infection-control precautions. Many hospitals have developed Ebola task forces with this in mind.
Updated information is available at www.cdc.gov/vhf/ebola/index.html.
Pregnant women are highly susceptible to Listeriosis
A nationwide food recall in mid-2014 prompted significant media attention to L monocytogenes, particularly its effect on pregnant women, who have an incidence of Listerial infection 13 times higher than the general population. Although maternal illness is relatively mild, ranging from a complete lack of symptoms to febrile diarrhea, there is an increased risk to the fetus or neonate of loss, preterm labor, neonatal sepsis, meningitis, and death. The perinatal mortality rate is 29%.
The mainstay of prevention during pregnancy is improved food safety and handling, as well as counseling of pregnant women to avoid unpasteurized soft cheeses, raw milk, and unwashed fruits and vegetables, and to avoid or heat thoroughly lunch meats and hot dogs.
When a pregnant woman is exposed to Listeria, management depends on the clinical scenario, as outlined by ACOG:
- Asymptomatic pregnant women do not require testing, treatment, or fetal surveillance. Any development of symptoms within 2 months may justify further evaluation, however.
- Pregnant women with mild gastro-intestinal or flulike symptoms but no fever also can be managed expectantly. Blood cultures may be appropriate; if positive, antibiotic therapy should be initiated.
- A febrile pregnant woman should have blood cultures assessed and be started on antibiotics. The preferred regimen is intravenous ampicillin 6 g/day with or without gentamicin for 14 days. If delivery occurs, placental cultures may be assessed. Listeriosis also can be diagnosed by amniocentesis. Stool cultures are not recommended.
Influenza is largely preventable
It is important to remember that one of the most dangerous viruses for pregnant women can be prevented. However, only 38% to 52% of women who should have received the influenza vaccine around the time of pregnancy actually did so between 2009 and 2013, according to the ACOG Committee Opinion cited above. Pregnant and postpartum women are at increased risk of serious illness, prolonged hospitalization, and death from influenza infection.
The vaccine is safe and effective. Not only does it prevent maternal morbidity and mortality, but it reduces neonatal complications. Inactivated vaccine is recommended for all pregnant women at any gestational age during the flu season.
Because many women are hesitant to accept the vaccine, accurate education is essential to dispel misconceptions about it and its components. It has been shown that if an obstetric clinician recommends the vaccine and makes it available, pregnant patients are five to 50 times more likely to receive it. As obstetricians, we are compelled to make this a priority in our practice.
What this EVIDENCE means for practice
Be alert and ready to act if an infectious threat is noted in your obstetric population. Get your flu shot. Give it to your obstetric patients. And don’t forget that ACOG also supports the administration of one dose of the tetanus, diphtheria, and pertussis vaccine during each pregnancy.
How much prenatal screening is too much?
Goetzinger KR, Odibo AO. Screening for abnormal placentation and adverse pregnancy outcomes with maternal serum biomarkers in the second trimester. Prenatal Diagn. 2014;34(7):635–641.
D’Antonio F, Rijo C, Thilaganathan B, et al. Association between first-trimester maternal serum pregnancy associated plasma protein-A and obstetric complications. Prenatal Diagn. 2013;33(9):839–847.
Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
Martin A, Krishna I, Martina B, et al. Can the quantity of cell-free fetal DNA predict preeclampsia: a systematic review. Prenatal Diagn. 2014;34(7): 685–691.
Audibert F, Boucoiran I, An N, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203(4):383.e1–e8.
Myatt L, Clifton RG, Roberts JM, et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242.
The placenta of a normal pregnancy secretes small amounts of a variety of biomarkers such as alpha-fetoprotein (AFP), human chorionic gonadotropin, unconjugated estriol, inhibin A, pregnancy-associated placental protein A (PAPP-A), soluble fms-like tyrosine kinase, and placental growth factor.
The association between abnormal maternal serum biomarkers and abnormal pregnancy outcomes has been known since the 1970s, when elevated AFP was noted in pregnancies with fetal open neural tube defects. Shortly thereafter, low levels of AFP were associated with fetuses with trisomy 21.
One theory is that the abnormality in pregnancy leads to abnormal regulation at the level of the fetal-placental interface and over- or under-secretion of the various biomarkers. An offshoot of this theory is the idea that abnormal placentation (ie, preeclampsia, fetal growth restriction, accreta) also may be reflected in elevated or suppressed secretion of placental biomarkers, which could be used to screen for these conditions during pregnancy.
PAPP-A is a placental serum marker that is a component of first-trimester genetic screening. It is a marker of placental function, and low levels have been associated with fetal growth restriction, preterm birth, preeclampsia, and fetal loss. Another first-trimester marker associated with adverse outcomes is cell-free fetal DNA. This DNA, found in the maternal blood, is a product of placental apoptosis, and elevated levels have been demonstrated in women who develop preeclampsia.
Although many of the biomarkers listed here are not available specifically as a clinical screening test in the United States, the link to common genetic screens makes it tempting to try to add prediction of preeclampsia and other information to an existing test. If specific numbers are reported on the genetic screen for the different markers, that information is already there, and some companies may flag abnormally high or low levels.
However, although the association between abnormal pregnancy outcomes and abnormal biomarkers is well established in the literature, the clinical predictive value is not—nor is there always an effective intervention available. One could argue that low-dose aspirin, which is already recommended for patients with a prior delivery before 34 weeks due to preeclampsia, or more than one prior pregnancy with preeclampsia, could be recommended for patients identified on early screens to be at increased risk for preeclampsia. This approach should be tested in randomized clinical trials before universal adoption.
What this EVIDENCE means for practice
Although it is tempting to use associations to predict adverse events, the clinical value of doing so has not yet been proven. Exercise caution before potentially causing concern for both you and your patient.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):1–67.
2. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Consortium on Safe Labor. Obstet Gynecol. 2010;116(6):1281–1287.
1. Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. Natl Vital Stat Rep. 2013;62(9):1–67.
2. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Consortium on Safe Labor. Obstet Gynecol. 2010;116(6):1281–1287.
IN THIS ARTICLE
— Is adherence to new labor curves the best way to reduce the rate of primary cesarean?
— Be vigilant for infectious threats to your obstetric population
— How much prenatal screening is too much?
Hypertension and pregnancy and preventing the first cesarean delivery
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
Have you read Dr. Errol R. Norwitz's Update on Operative Vaginal Delivery? Click here to access.
A new paradigm for preeclampsia
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
That preeclampsia is a growing problem, and one of the most significant causes of maternal-fetal morbidity and mortality today, is what drove the American College of Obstetricians and Gynecologists (ACOG) to convene a task force on hypertension in pregnancy in 2011. Indeed, the incidence of preeclampsia has increased approximately 10% over the last 3 decades in the United States, such that approximately 5%-7% of all pregnant women will develop the disorder.
The specific etiology of preeclampsia remains unclear, but the reasons for the increased incidence likely include the rise in delayed childbearing, the increased use of assisted reproductive technology, a rise in the number of twins, and the obesity pandemic.
Epidemiologically, older women with their first pregnancy are at higher risk of developing preeclampsia, whether or not they use assisted reproductive technology (ART). The not-infrequent use of ART among older women, namely IVF, has a compounding effect. So too, does the incidence of twinning. While we fortunately are seeing a lower rate of higher-order multiple gestations associated with IVF than we did in the 1990s, the incidence of twinning has increased dramatically. Women with multiple gestations of any order are at higher risk of developing preeclampsia.
The obesity pandemic is widely believed to be the most modifiable risk factor for preeclampsia. If we can help women to achieve a body mass index (BMI) that is as close to optimal as possible prior to conception, we will likely see significant reductions in the incidence of hypertensive disorders.
Prompt diagnosis of preeclampsia is critical, and on this front, ACOG\'s Task Force on Hypertension in Pregnancy report of 2013 sets forth an important new paradigm for thinking about the disorder and establishing its presence.
Managing preeclampsia remains challenging, however, as there are many areas in which evidence for guiding therapy and management is still insufficient. ACOG’s task force set out to review available data and to attempt to provide clarity on the management of preeclampsia as well as its diagnosis. This was no easy task, and in their culminating report, which lists 60 distinct recommendations, the task force clearly acknowledges the weak evidence base, giving relatively few of their recommendations top marks for both the quality of evidence and their strength of recommendation.
The report appropriately reminds us that there are few if any prescriptions or protocols when it comes to managing preeclampsia. My main concern with the task force’s coverage of management involves their recommendations that magnesium sulfate be used to treat patients with eclampsia and those with preeclampsia with severe features, but not necessarily those without severe features. Patients can progress so rapidly that unless every woman with preeclampsia is vigilantly scrutinized during labor and post delivery – a difficult, if not impossible, task – the window of opportunity to prevent convulsions through the use of magnesium sulfate may well be missed.
ACOG’s new terminology, definitions
Importantly, ACOG’s Task Force on Hypertension in Pregnancy report emphasizes that preeclampsia is an evolving, dynamic, and multisystemic process. It recommends elimination of the terms "mild" and "severe" preeclampsia and encourages the use of new terminology, pushing us to think instead of preeclampsia as being a disorder with or without "severe features." According to the report, a diagnosis of "mild preeclampsia" applies only at the moment at which the diagnosis is established, making the phrase misleading.
Physicians and other providers who have long been in practice will have a hard time ridding their vocabulary of the terms mild and severe preeclampsia, but the intent of the recommendation – to foster appreciation of preeclampsia as an evolving disease – is important and should become entrenched in our approach to hypertension in pregnancy.
The report also downgrades the role of proteinuria in the diagnosis of preeclampsia. Proteinuria is defined as the excretion of 300 mg or more of protein in a 24-hour urine collection or a urine protein/creatinine ratio of at least 0.3 mg/dL. Although proteinuria may indeed be a primary diagnostic finding, it should not be required in order to make the diagnosis of preeclampsia if other severe features are present.
As described in the report, severe features of preeclampsia may include thrombocytopenia (platelet count less than 100,000/microliter), impaired liver function, a rise in serum creatinine indicating progressive renal insufficiency (a serum creatinine concentration greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease), central nervous system disturbances, pulmonary edema, and persistently high elevations in blood pressure (a systolic blood pressure of 160 mm Hg or higher or a diastolic reading of 110 mm Hg or higher on two occasions at least 4 hours apart).
The document states, in other words, that to establish preeclampsia, equal weight should be given to any of these so-called severe features – even in the absence of proteinuria – when they occur along with new-onset hypertension at 20 weeks’ gestation or beyond. (The blood pressure criteria stipulate hypertension as a systolic blood pressure of 140 mm Hg or higher and a diastolic of 90 mm Hg or higher, taken on at least two occasions 4 hours apart.)
This new approach to diagnosing preeclampsia is a major change. It reflects what the ACOG document rightly calls a "minimal relationship" between the quantity of urinary protein and maternal and fetal outcomes in preeclampsia, as well as the fact that preeclampsia may quickly evolve. We need these broader criteria and lower thresholds so that we will not miss patients who might present atypically, with proteinuria not yet a significant finding, but with disease quickly developing.
Another helpful change is the removal of significant fetal growth restriction (less than the fifth percentile) as a diagnostic feature for traditionally coined "severe preeclampsia." Fetal growth restriction is not included in ACOG’s recommended list of severe features of preeclampsia. This is a helpful clarification that should prevent potentially unnecessary deliveries. The problems of fetal growth restriction (FGR) and preeclampsia should be considered and managed separately, with the option of expectant management considered with respect to each entity alone, as opposed to the finding of FGR driving a diagnosis of severe preeclampsia and thus delivery within 48 hours.
Where evidence is strongest
ACOG’s report contains 60 distinct recommendations covering hypertensive disorders in pregnancy, but only 6 of these recommendations are graded as both "strong" and based on "high-quality" evidence. (A "strong" recommendation is one that the task force considered so well supported by the literature that it is applicable to "virtually all patients.")
The remaining recommendations are either graded as "qualified" or are based on evidence that is rated as "very low," "low," or "moderate," or some combination of the "qualified" grade and a quality-of-evidence ranking below "high."
The task force utilized a strategy developed by the Grading of Recommendations Assessment, Development and Evaluation Working Group that rates the quality of evidence based largely on what’s called "confidence in estimates of effect." Under this approach, randomized, controlled trials are important but may still be considered flawed and observational studies are usually but not necessarily classified as low quality.
The small number of strong, high-quality recommendations in the ACOG report reflects the fact that, despite advances in our understanding of preeclampsia, there are many areas in which we lack good evidence from randomized, controlled trials. Importantly, the task force emphasizes that it offers recommendations and not prescriptions, and that sound clinical judgment remains a key part of patient management.
The six strong recommendations in the report that are based on high-quality evidence are as follows:
• The administration of vitamin C or vitamin E to prevent preeclampsia is not recommended.
• Women with severe preeclampsia who are being expectantly managed at 34 weeks’ or less of gestation should receive corticosteroids for the benefit of accelerating fetal lung maturation.
• Women who have chronic hypertension with superimposed preeclampsia and are being expectantly managed at 34 weeks’ or less of gestation also should have corticosteroids administered for the purpose of accelerating fetal lung maturation.
• For women with eclampsia, the administration of parenteral magnesium sulfate is recommended.
• For women with severe preeclampsia, the administration of intrapartum-postpartum magnesium sulfate to prevent eclampsia is recommended.
• For women with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome who are before the gestational age of fetal viability, delivery should be undertaken shortly after initial maternal stabilization.
My take
Contrary to former recommendations, the task force’s new recommendations suggest that use of magnesium sulfate for preeclampsia without severe features (formerly called "mild preeclampsia") may not be needed. Although magnesium sulfate may not be warranted in every case, patients can progress so rapidly from having no severe symptoms to developing severe symptoms that it is difficult if not impossible to parse out who would or would not benefit from treatment. If we try to do so, we run the great risk of not providing the necessary medication to our patients, thereby increasing the chances of maternal morbidity and mortality.
In our institution, we continue to use magnesium sulfate intrapartum and post partum for every patient with a diagnosis of preeclampsia, whether or not she has severe features. Without high-quality evidence to the contrary, I do not believe that we should alter the former recommendation that magnesium sulfate be used in all cases of preeclampsia. We administer the agent for 24 hours post partum because studies looking at a 48-hour window have shown that most patients who have an eclamptic seizure within 48 hours after delivery will actually experience it within the first 24 hours.
There is a remaining conundrum. We know that approximately 50% of postpartum seizures occur more than 48 hours post delivery. It is vital, therefore, that patients who have hypertensive disorders during pregnancy be educated about the signs and symptoms of postpartum preeclampsia and be given clear directions about how to contact their provider if any signs or symptoms – such as headache, visual disturbances, and right-upper-quadrant pain – are noticed. Patients who are discharged with blood pressures that are still elevated (but not enough to require hospitalization) should be seen again within 72 hours or a week for an evaluation of their blood pressure. The threshold in our practices for arranging earlier postpartum visits, moreover, should be set very low for these women.
The need for patient education is mentioned in the ACOG report, which says, "It is suggested that health care providers convey information about preeclampsia in the context of prenatal care and postpartum care using proven health care communication practices."
The wording of the recommendation as well as its "qualified" strength and the designation of a "low" quality of evidence should not detract from the importance of the message that patient education is a key to successful recognition and management of preeclampsia. The awareness of and knowledge about postpartum preeclampsia have been shown in recent research to be disappointingly low. We need to do better.
With respect to prevention, there are two strategies that, despite not having strong recommendations and/or the backing of high-quality evidence, are still considered to be effective. One is the use of daily low-dose aspirin (60-80 mg) in women with a history of early-onset preeclampsia and preterm delivery prior to 34 weeks’ of gestation. The other is calcium supplementation for women who have an inadequate daily intake of calcium, although this practice has less relevance in the United States than in the developing world.
The goal of low-dose aspirin is really to reduce the likelihood of severe preeclampsia recurring. Almost half of patients with a history of preeclampsia will not develop the disorder in a subsequent pregnancy (without aspirin therapy), so the reduction in the incidence of preeclampsia with low-dose aspirin is unlikely to be significant. However, as aspirin therapy is a relatively benign and inexpensive intervention, it is worth considering. Indeed, meta-analyses of women in randomized trials of low-dose aspirin for preeclampsia prevention have shown small reductions in the incidence and morbidity of preeclampsia, without any evidence of adverse effects.
[Notably, the U.S. Preventive Services Task Force said in a draft recommendation statement issued in April that it recommends low-dose aspirin use after 12 weeks of pregnancy in women who are at high risk for preeclampsia. (See accompanying story.)]
Over the years a variety of predictive strategies – for predicting early-onset preeclampsia in particular – have been proposed and researched, including various biomarkers, blood tests, and imaging studies. These are all worthwhile endeavors at the research level, but for any screening strategy to be implemented there must be an effective intervention. At the current time, our only effective intervention is delivery based on clinical findings.
On the other end of the spectrum, it is clear today that preeclampsia is associated with later-life cardiovascular disease in women. It is only in the last 5-10 years that research findings have come to the fore and that we have thought about preeclampsia as a marker for increased disease risk, just as we did starting several decades ago with gestational diabetes and the risk of future type 2 diabetes.
ACOG’s task force suggests yearly assessment of blood pressure, lipids, fasting blood glucose, and BMI in women with a medical history of preeclampsia who delivered preterm or who have a history of recurrent preeclampsia. The recommendation is "qualified," with a low quality of evidence, and contains a cautionary footnote stating that "the value and appropriate timing of assessment is not yet established."
Indeed, the next step in research will be to follow patients with preeclampsia longitudinally and determine whether or not interventional strategies can be devised to reduce the cardiovascular risk to baseline or, ideally below baseline, in these patients. Until we have such information, we should still recognize that this group of patients may be at higher risk for cardiovascular disease later in life, and, at the very least, be even more vigilant about adhering to regular health maintenance examinations.
Dr. John T. Repke is professor and chair of obstetrics and gynecology at Pennsylvania State University, Hershey, and has published extensively on hypertension in pregnancy. Dr. Repke said he has no relevant financial disclosures.
Update on Obstetrics
Over the past 20 years, the incidence of preeclampsia in the United States has increased 25%,1 and the disorder is a leading cause of morbidity and death among both mothers and infants. Although considerable progress has been achieved in elucidating the pathophysiology of preeclampsia, greater understanding has not yet carried over into improved clinical practice.
To address this disconnect between data and practice, the American College of Obstetricians and Gynecologists (ACOG) issued a 99-page document in November 2013 to help establish best practices in the diagnosis and management of hypertensive disorders in pregnancy. We begin this article with a look at its major recommendations.
Other notable developments in obstetrics over the past year have been the rapid evolution of noninvasive prenatal testing and the publication of new guidance on screening, diagnosis, and management of gestational diabetes, all of which are addressed in this article.
ACOG AIMS TO CLARIFY BEST PRACTICES IN THE MANAGEMENT OF HYPERTENSION IN PREGNANCY
American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
The biggest news of the past year is probably the November 2013 report on hypertension in pregnancy from ACOG, which was developed with three goals in mind:
to summarize current knowledge
to provide best-practice guidelines
to identify areas in which further research is needed.
The classification, diagnosis, prediction, prevention, and management of gestational hypertension, preeclampsia, and chronic hypertension are addressed in the report. Although space constraints prevent us from summarizing the entire document, we would like to highlight the biggest changes and most relevant additions to clinical practice.
Notable recommendations
Classification. Preeclampsia is no longer characterized as “mild” or “severe” but as “preeclampsia without severe features” and “preeclampsia with severe features.” As justification for these changes, the ACOG Task Force on Hypertension in Pregnancy noted that preeclampsia is progressive by nature, so a characterization of “mild” disease is appropriate only at the time of diagnosis. Therefore, “appropriate management mandates frequent reevaluation for severe features.”
Diagnosis of proteinuria. The options are a 24-hour urine collection demonstrating more than 300 mg of protein or a single-specimen urine protein:creatinine ratio of 0.3 mg/dL or higher. Dipstick values should only be used if these quantitative measures are unavailable.
Signs of severe disease. Fetal growth restriction and proteinuria of more than 5 g/24 hr are no longer considered defining features of severe disease.
Severe features now include any of these:
systolic blood pressure (BP) of 160 mm Hg or higher, or diastolic BP of 110 mm Hg or higher on two occasions at least 4 hours apart while the patient is on bed rest (unless antihypertensive therapy is initiated before this time)
thrombocytopenia (platelets <100 x 109/L)
impaired liver function, as indicated by abnormally elevated blood concentrations of liver enzymes (to twice normal concentration) and/or severe, persistent right upper quadrant or epigastric pain unresponsive to medication and not accounted for by alternative diagnoses
progressive renal insufficiency (serum creatinine concentration >1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease)
pulmonary edema
new-onset visual or central nervous system disturbances.
Related Article: Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia? George Macones, MD (Examining the Evidence, December 2012)
Screening for preeclampsia. The use of Doppler studies and serum biomarkers is not recommended, as there is no evidence that early identification translates to improved outcomes.
Prevention of preeclampsia. Low-dose aspirin (60–80 mg/d, starting in the late first trimester) should be offered as primary prevention to:
women with a history of early-onset preeclampsia and delivery before 34 weeks’ gestation
women with a history of preeclampsia in multiple pregnancies
other high-risk patients (chronic hypertension, diabetes).
No other treatments (vitamin C or E, salt restriction, or bed rest) are recommended for the prevention of preeclampsia, although calcium supplementation may be recommended for women with a low baseline dietary intake of calcium.
Use of magnesium sulfate. Universal prophylaxis with magnesium sulfate is not recommended for preeclampsia unless severe features are present or the patient’s clinical condition changes to severe during labor.
Timing of delivery. Recommendations for delivery for patients with hypertensive disorders are:
gestational hypertension or preeclampsia without severe features: 37 weeks’ gestation
preeclampsia with severe features: by 34 weeks
chronic hypertension: not before 38 weeks
chronic hypertension with superimposed preeclampsia: 34 or 37 weeks, depending on the presence of severe features.
All of these recommendations are contingent upon the clinical status of the patient and her fetus. For example, if the fetus develops severe growth restriction (<5%) or oligohydramnios, delivery may be recommended regardless of gestational age, based on fetal testing and maternal stability.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai (October 2013)
Postpartum hypertension. The need for recognition of hypertension in the postpartum period is emphasized, as well as appropriate management, using the following guidelines:
Be aware that BP decreases initially after delivery and then increases 3 to 6 days postpartum, requiring vigilance on the part of the clinician. For this reason, BP monitoring is recommended 72 hours postpartum (inpatient or outpatient) and again in 7 to 10 days in women diagnosed with a hypertensive disorder of pregnancy.
Counsel patients who experience a hypertensive disorder and/or preeclampsia during pregnancy about postpartum preeclampsia, providing strict precautions and explicit instructions regarding its signs and symptoms
If BP remains elevated after the first postpartum day, consider discontinuing nonsteroidal anti-inflammatory drugs (NSAIDs), as they may be related to hypertension
Treat BP that remains above 150/100 mm Hg with antihypertensive therapy
If postpartum preeclampsia is suspected, administer magnesium sulfate for 24 hours.
A culture shift is needed
At our institution, the most significant potential changes to clinical practice may be the elimination of universal magnesium sulfate prophylaxis and the removal of severe fetal growth restriction from the definition of “severe” preeclampsia.
Also, because the use of NSAIDs is widespread for postpartum pain control, a culture change is needed if we are to follow the postpartum recommendations.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although we suggest that you read the ACOG report thoroughly, remember that its recommendations are only that—recommendations. Also keep in mind that only six of the approximately 60 “recommendations” provided in this report were accompanied by both high-quality evidence and a strong recommendation. There still is room for clinical judgment and individualization of management to specific patient populations.
NONINVASIVE PRENATAL SCREENING IS EXPANDING RAPIDLY—BUT DON’T THROW OUT THAT CVS KIT JUST YET!
ACOG Committee on Genetics.Committee Opinion #545: Noninvasive prenatal testing for fetal aneuploidy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120(6):1532–1534.
Mennuti MT, Cherry AM, Morrissette JJD, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy? Am J Obstet Gynecol. 2013;209(5):415–419.
In last year’s Update in Obstetrics, we discussed noninvasive prenatal genetic screening via cell-free fetal DNA, noting that it is a safer (no risk of miscarriage) and faster (starting at 10 weeks’ gestation) way to screen for aneuploidy. The sensitivity and specificity of this test for Trisomy 21 and 18 are over 99%, with slightly lower sensitivity for Trisomy 13 and sex chromosome abnormalities and a false-positive rate of 0.5%.
In a committee opinion published in December 2012, ACOG concluded that
cell-free fetal DNA is an appropriate screening option only for specific groups of patients at risk for aneuploidy:
women older than age 35
women with fetal ultrasonography findings that are concerning for aneuploidy
women with aneuploidy in a prior pregnancy
women with abnormal first- or second-trimester genetic screening tests
parents with a balanced translocation and an increased risk of Trisomy 21 or 13.
Noninvasive aneuploidy testing is for screening only
Negative results are not diagnostic, and all positive results should be confirmed with invasive testing (chorionic villus sampling [CVS] or amniocentesis).
ACOG does not recommend routine use of cell-free fetal DNA without a comprehensive history and adequate patient counseling, as well as a designation of “high risk.”
Over the past year, more options have become available for aneuploidy screening via cell-free fetal DNA, including screening in twin gestations (for Trisomy 21, 18, 13, and the presence of a Y chromosome only) and screening for:
22q deletion (DiGeorge syndrome)
5p– (Cri-du-chat syndrome)
15q (Prader-Willi and Angelman syndromes)
1p (1p36 deletion syndrome)
Trisomy 16
Trisomy 22.
At this rate, the genetic information potentially available via noninvasive testing seems unlimited. It is easy to see how the fact that this is a screening test—not a diagnostic test—could get lost in the excitement.
Other limitations: Noninvasive testing is still not validated in low-risk patients, and the false-positive risk may be higher than original estimates.
Related Article: Noninvasive prenatal DNA testing: A survey of who is using it, and how (Audiocast, June 2013)
The problem of false positives
This issue was addressed by Mennuti and colleagues, who presented eight cases of abnormal cell-free fetal DNA results that were not confirmed by invasive testing.
There are few prospective data about the source of false-positive results; potential mechanisms include an inadequate fetal fraction of cell-free DNA, maternal or placental mosaicism, and a vanishing twin.
Mennuti and colleagues propose that a registry of false-positive and false-negative results be established to gather further data. They also note that as low-risk patients and aneuploidies of lower and lower prevalence are incorporated into noninvasive testing, the false-positive rate will rise. Their findings have implications for patient counseling, patient distress, invasive testing, and reimbursement.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Cell-free fetal DNA is a rapidly expanding screening technology, but it is not ready to replace diagnostic testing. Don’t throw away those CVS and amniocentesis kits just yet—we are a long way from a completely noninvasive world.
WHEN IT COMES TO GESTATIONAL DIABETES, LESS MAY BE MORE
ACOG Committee on Practice Bulletins–Obstetrics. Practice Bulletin #137: Gestational diabetes mellitus. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122(2 Pt 1):406–416.
Gestational diabetes accounts for 90% of diabetic pregnancies, and its incidence has been increasing in the United States along with the obesity epidemic. In recent years, there has been some debate about the best way to screen for, diagnose, and treat gestational diabetes.
Multiple criteria exist for a positive 1-hour (130–140 mg/dL) or 3-hour glucose tolerance test (Carpenter and Coustan vs National Diabetes Data Group), without comparative trials or consensus as to which version is best. Lower cutoffs increase the rate of gestational diabetes by as much as 50%, whereas higher cutoffs lower the false-positive rate and reduce the need for additional tests.
Some groups have recommended moving away from the traditional two-step process to a one-step approach that utilizes the 2-hour, 75-g glucose tolerance test commonly used outside of pregnancy. They argue that this approach would simplify and standardize the process and could improve outcomes in “borderline” pregnancies that would have been missed by less stringent guidelines.
However, the baseline rate of gestational diabetes using this one-step approach would likely increase from 7% to 18% or higher, depending on the patient population. Such an increase would trigger a huge rise in costs and resources needed to care for these patients, without data on outcomes or appropriate therapy for this expanded group of women with gestational diabetes.
Treatment isn't clear-cut, either
Treatment of gestational diabetes centers on labor-intensive glucose monitoring, nutritional interventions, and insulin therapy.
Until recently, the use of oral hypoglycemic agents was not recommended due to limited data. Multiple studies now have been performed to evaluate the safety and efficacy of glyburide and metformin in pregnancy, demonstrating glucose control similar to that achieved with insulin without short-term adverse effects in the mother or newborn. However, as many as 20% to 40% of women using glyburide and 50% of those using metformin require the addition of insulin for adequate glucose control. The long-term effects of these medications are unknown.
Related Article: Does myo-inositol supplementation reduce the rate of gestational diabetes in pregnant women with a family history of type 2 diabetes? E. Albert Reece, MD, PhD, MBA (Examining the Evidence, June 2013)
ACOG weighs in
In an attempt to clarify optimal screening, diagnosis, and treatment, ACOG updated its practice bulletin on gestational diabetes in August 2013. Among its recommendations:
Avoid the 2-hour glucose tolerance test because there is no demonstrated benefit for the increased number of mothers (and their fetuses) that would be identified by this approach. Rather, use the two-step approach of a 1-hour 50-g glucose tolerance test followed by a 3-hour 100-g glucose tolerance test.
In regard to the 1-hour test, ACOG finds either 135 or 140 mg/dL acceptable as a cutoff but recommends that each practice choose one value as a standard and use it consistently. For the 3-hour test, ACOG recommends that each practice choose the version that best fits its population and prevalence of diabetes. At our institution, for example, we have chosen a 1-hour cutoff of 140 mg/dL and the National Diabetes Data Group criteria for the 3-hour test (105, 190, 165, and 145 mg/dL).
Oral glyburide or metformin may be used to treat gestational diabetes, but glyburide (starting at 2.5 mg/d) may be the better choice for glucose control.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use the two-step screening process to identify gestational diabetes, picking your cutoffs and sticking to them. Oral hypoglycemic agents now are acceptable for treatment, but be prepared to go back to insulin if necessary.
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526.
Over the past 20 years, the incidence of preeclampsia in the United States has increased 25%,1 and the disorder is a leading cause of morbidity and death among both mothers and infants. Although considerable progress has been achieved in elucidating the pathophysiology of preeclampsia, greater understanding has not yet carried over into improved clinical practice.
To address this disconnect between data and practice, the American College of Obstetricians and Gynecologists (ACOG) issued a 99-page document in November 2013 to help establish best practices in the diagnosis and management of hypertensive disorders in pregnancy. We begin this article with a look at its major recommendations.
Other notable developments in obstetrics over the past year have been the rapid evolution of noninvasive prenatal testing and the publication of new guidance on screening, diagnosis, and management of gestational diabetes, all of which are addressed in this article.
ACOG AIMS TO CLARIFY BEST PRACTICES IN THE MANAGEMENT OF HYPERTENSION IN PREGNANCY
American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
The biggest news of the past year is probably the November 2013 report on hypertension in pregnancy from ACOG, which was developed with three goals in mind:
to summarize current knowledge
to provide best-practice guidelines
to identify areas in which further research is needed.
The classification, diagnosis, prediction, prevention, and management of gestational hypertension, preeclampsia, and chronic hypertension are addressed in the report. Although space constraints prevent us from summarizing the entire document, we would like to highlight the biggest changes and most relevant additions to clinical practice.
Notable recommendations
Classification. Preeclampsia is no longer characterized as “mild” or “severe” but as “preeclampsia without severe features” and “preeclampsia with severe features.” As justification for these changes, the ACOG Task Force on Hypertension in Pregnancy noted that preeclampsia is progressive by nature, so a characterization of “mild” disease is appropriate only at the time of diagnosis. Therefore, “appropriate management mandates frequent reevaluation for severe features.”
Diagnosis of proteinuria. The options are a 24-hour urine collection demonstrating more than 300 mg of protein or a single-specimen urine protein:creatinine ratio of 0.3 mg/dL or higher. Dipstick values should only be used if these quantitative measures are unavailable.
Signs of severe disease. Fetal growth restriction and proteinuria of more than 5 g/24 hr are no longer considered defining features of severe disease.
Severe features now include any of these:
systolic blood pressure (BP) of 160 mm Hg or higher, or diastolic BP of 110 mm Hg or higher on two occasions at least 4 hours apart while the patient is on bed rest (unless antihypertensive therapy is initiated before this time)
thrombocytopenia (platelets <100 x 109/L)
impaired liver function, as indicated by abnormally elevated blood concentrations of liver enzymes (to twice normal concentration) and/or severe, persistent right upper quadrant or epigastric pain unresponsive to medication and not accounted for by alternative diagnoses
progressive renal insufficiency (serum creatinine concentration >1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease)
pulmonary edema
new-onset visual or central nervous system disturbances.
Related Article: Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia? George Macones, MD (Examining the Evidence, December 2012)
Screening for preeclampsia. The use of Doppler studies and serum biomarkers is not recommended, as there is no evidence that early identification translates to improved outcomes.
Prevention of preeclampsia. Low-dose aspirin (60–80 mg/d, starting in the late first trimester) should be offered as primary prevention to:
women with a history of early-onset preeclampsia and delivery before 34 weeks’ gestation
women with a history of preeclampsia in multiple pregnancies
other high-risk patients (chronic hypertension, diabetes).
No other treatments (vitamin C or E, salt restriction, or bed rest) are recommended for the prevention of preeclampsia, although calcium supplementation may be recommended for women with a low baseline dietary intake of calcium.
Use of magnesium sulfate. Universal prophylaxis with magnesium sulfate is not recommended for preeclampsia unless severe features are present or the patient’s clinical condition changes to severe during labor.
Timing of delivery. Recommendations for delivery for patients with hypertensive disorders are:
gestational hypertension or preeclampsia without severe features: 37 weeks’ gestation
preeclampsia with severe features: by 34 weeks
chronic hypertension: not before 38 weeks
chronic hypertension with superimposed preeclampsia: 34 or 37 weeks, depending on the presence of severe features.
All of these recommendations are contingent upon the clinical status of the patient and her fetus. For example, if the fetus develops severe growth restriction (<5%) or oligohydramnios, delivery may be recommended regardless of gestational age, based on fetal testing and maternal stability.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai (October 2013)
Postpartum hypertension. The need for recognition of hypertension in the postpartum period is emphasized, as well as appropriate management, using the following guidelines:
Be aware that BP decreases initially after delivery and then increases 3 to 6 days postpartum, requiring vigilance on the part of the clinician. For this reason, BP monitoring is recommended 72 hours postpartum (inpatient or outpatient) and again in 7 to 10 days in women diagnosed with a hypertensive disorder of pregnancy.
Counsel patients who experience a hypertensive disorder and/or preeclampsia during pregnancy about postpartum preeclampsia, providing strict precautions and explicit instructions regarding its signs and symptoms
If BP remains elevated after the first postpartum day, consider discontinuing nonsteroidal anti-inflammatory drugs (NSAIDs), as they may be related to hypertension
Treat BP that remains above 150/100 mm Hg with antihypertensive therapy
If postpartum preeclampsia is suspected, administer magnesium sulfate for 24 hours.
A culture shift is needed
At our institution, the most significant potential changes to clinical practice may be the elimination of universal magnesium sulfate prophylaxis and the removal of severe fetal growth restriction from the definition of “severe” preeclampsia.
Also, because the use of NSAIDs is widespread for postpartum pain control, a culture change is needed if we are to follow the postpartum recommendations.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although we suggest that you read the ACOG report thoroughly, remember that its recommendations are only that—recommendations. Also keep in mind that only six of the approximately 60 “recommendations” provided in this report were accompanied by both high-quality evidence and a strong recommendation. There still is room for clinical judgment and individualization of management to specific patient populations.
NONINVASIVE PRENATAL SCREENING IS EXPANDING RAPIDLY—BUT DON’T THROW OUT THAT CVS KIT JUST YET!
ACOG Committee on Genetics.Committee Opinion #545: Noninvasive prenatal testing for fetal aneuploidy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120(6):1532–1534.
Mennuti MT, Cherry AM, Morrissette JJD, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy? Am J Obstet Gynecol. 2013;209(5):415–419.
In last year’s Update in Obstetrics, we discussed noninvasive prenatal genetic screening via cell-free fetal DNA, noting that it is a safer (no risk of miscarriage) and faster (starting at 10 weeks’ gestation) way to screen for aneuploidy. The sensitivity and specificity of this test for Trisomy 21 and 18 are over 99%, with slightly lower sensitivity for Trisomy 13 and sex chromosome abnormalities and a false-positive rate of 0.5%.
In a committee opinion published in December 2012, ACOG concluded that
cell-free fetal DNA is an appropriate screening option only for specific groups of patients at risk for aneuploidy:
women older than age 35
women with fetal ultrasonography findings that are concerning for aneuploidy
women with aneuploidy in a prior pregnancy
women with abnormal first- or second-trimester genetic screening tests
parents with a balanced translocation and an increased risk of Trisomy 21 or 13.
Noninvasive aneuploidy testing is for screening only
Negative results are not diagnostic, and all positive results should be confirmed with invasive testing (chorionic villus sampling [CVS] or amniocentesis).
ACOG does not recommend routine use of cell-free fetal DNA without a comprehensive history and adequate patient counseling, as well as a designation of “high risk.”
Over the past year, more options have become available for aneuploidy screening via cell-free fetal DNA, including screening in twin gestations (for Trisomy 21, 18, 13, and the presence of a Y chromosome only) and screening for:
22q deletion (DiGeorge syndrome)
5p– (Cri-du-chat syndrome)
15q (Prader-Willi and Angelman syndromes)
1p (1p36 deletion syndrome)
Trisomy 16
Trisomy 22.
At this rate, the genetic information potentially available via noninvasive testing seems unlimited. It is easy to see how the fact that this is a screening test—not a diagnostic test—could get lost in the excitement.
Other limitations: Noninvasive testing is still not validated in low-risk patients, and the false-positive risk may be higher than original estimates.
Related Article: Noninvasive prenatal DNA testing: A survey of who is using it, and how (Audiocast, June 2013)
The problem of false positives
This issue was addressed by Mennuti and colleagues, who presented eight cases of abnormal cell-free fetal DNA results that were not confirmed by invasive testing.
There are few prospective data about the source of false-positive results; potential mechanisms include an inadequate fetal fraction of cell-free DNA, maternal or placental mosaicism, and a vanishing twin.
Mennuti and colleagues propose that a registry of false-positive and false-negative results be established to gather further data. They also note that as low-risk patients and aneuploidies of lower and lower prevalence are incorporated into noninvasive testing, the false-positive rate will rise. Their findings have implications for patient counseling, patient distress, invasive testing, and reimbursement.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Cell-free fetal DNA is a rapidly expanding screening technology, but it is not ready to replace diagnostic testing. Don’t throw away those CVS and amniocentesis kits just yet—we are a long way from a completely noninvasive world.
WHEN IT COMES TO GESTATIONAL DIABETES, LESS MAY BE MORE
ACOG Committee on Practice Bulletins–Obstetrics. Practice Bulletin #137: Gestational diabetes mellitus. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122(2 Pt 1):406–416.
Gestational diabetes accounts for 90% of diabetic pregnancies, and its incidence has been increasing in the United States along with the obesity epidemic. In recent years, there has been some debate about the best way to screen for, diagnose, and treat gestational diabetes.
Multiple criteria exist for a positive 1-hour (130–140 mg/dL) or 3-hour glucose tolerance test (Carpenter and Coustan vs National Diabetes Data Group), without comparative trials or consensus as to which version is best. Lower cutoffs increase the rate of gestational diabetes by as much as 50%, whereas higher cutoffs lower the false-positive rate and reduce the need for additional tests.
Some groups have recommended moving away from the traditional two-step process to a one-step approach that utilizes the 2-hour, 75-g glucose tolerance test commonly used outside of pregnancy. They argue that this approach would simplify and standardize the process and could improve outcomes in “borderline” pregnancies that would have been missed by less stringent guidelines.
However, the baseline rate of gestational diabetes using this one-step approach would likely increase from 7% to 18% or higher, depending on the patient population. Such an increase would trigger a huge rise in costs and resources needed to care for these patients, without data on outcomes or appropriate therapy for this expanded group of women with gestational diabetes.
Treatment isn't clear-cut, either
Treatment of gestational diabetes centers on labor-intensive glucose monitoring, nutritional interventions, and insulin therapy.
Until recently, the use of oral hypoglycemic agents was not recommended due to limited data. Multiple studies now have been performed to evaluate the safety and efficacy of glyburide and metformin in pregnancy, demonstrating glucose control similar to that achieved with insulin without short-term adverse effects in the mother or newborn. However, as many as 20% to 40% of women using glyburide and 50% of those using metformin require the addition of insulin for adequate glucose control. The long-term effects of these medications are unknown.
Related Article: Does myo-inositol supplementation reduce the rate of gestational diabetes in pregnant women with a family history of type 2 diabetes? E. Albert Reece, MD, PhD, MBA (Examining the Evidence, June 2013)
ACOG weighs in
In an attempt to clarify optimal screening, diagnosis, and treatment, ACOG updated its practice bulletin on gestational diabetes in August 2013. Among its recommendations:
Avoid the 2-hour glucose tolerance test because there is no demonstrated benefit for the increased number of mothers (and their fetuses) that would be identified by this approach. Rather, use the two-step approach of a 1-hour 50-g glucose tolerance test followed by a 3-hour 100-g glucose tolerance test.
In regard to the 1-hour test, ACOG finds either 135 or 140 mg/dL acceptable as a cutoff but recommends that each practice choose one value as a standard and use it consistently. For the 3-hour test, ACOG recommends that each practice choose the version that best fits its population and prevalence of diabetes. At our institution, for example, we have chosen a 1-hour cutoff of 140 mg/dL and the National Diabetes Data Group criteria for the 3-hour test (105, 190, 165, and 145 mg/dL).
Oral glyburide or metformin may be used to treat gestational diabetes, but glyburide (starting at 2.5 mg/d) may be the better choice for glucose control.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use the two-step screening process to identify gestational diabetes, picking your cutoffs and sticking to them. Oral hypoglycemic agents now are acceptable for treatment, but be prepared to go back to insulin if necessary.
WE WANT TO HEAR FROM YOU. Tell us what you think.
Over the past 20 years, the incidence of preeclampsia in the United States has increased 25%,1 and the disorder is a leading cause of morbidity and death among both mothers and infants. Although considerable progress has been achieved in elucidating the pathophysiology of preeclampsia, greater understanding has not yet carried over into improved clinical practice.
To address this disconnect between data and practice, the American College of Obstetricians and Gynecologists (ACOG) issued a 99-page document in November 2013 to help establish best practices in the diagnosis and management of hypertensive disorders in pregnancy. We begin this article with a look at its major recommendations.
Other notable developments in obstetrics over the past year have been the rapid evolution of noninvasive prenatal testing and the publication of new guidance on screening, diagnosis, and management of gestational diabetes, all of which are addressed in this article.
ACOG AIMS TO CLARIFY BEST PRACTICES IN THE MANAGEMENT OF HYPERTENSION IN PREGNANCY
American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
The biggest news of the past year is probably the November 2013 report on hypertension in pregnancy from ACOG, which was developed with three goals in mind:
to summarize current knowledge
to provide best-practice guidelines
to identify areas in which further research is needed.
The classification, diagnosis, prediction, prevention, and management of gestational hypertension, preeclampsia, and chronic hypertension are addressed in the report. Although space constraints prevent us from summarizing the entire document, we would like to highlight the biggest changes and most relevant additions to clinical practice.
Notable recommendations
Classification. Preeclampsia is no longer characterized as “mild” or “severe” but as “preeclampsia without severe features” and “preeclampsia with severe features.” As justification for these changes, the ACOG Task Force on Hypertension in Pregnancy noted that preeclampsia is progressive by nature, so a characterization of “mild” disease is appropriate only at the time of diagnosis. Therefore, “appropriate management mandates frequent reevaluation for severe features.”
Diagnosis of proteinuria. The options are a 24-hour urine collection demonstrating more than 300 mg of protein or a single-specimen urine protein:creatinine ratio of 0.3 mg/dL or higher. Dipstick values should only be used if these quantitative measures are unavailable.
Signs of severe disease. Fetal growth restriction and proteinuria of more than 5 g/24 hr are no longer considered defining features of severe disease.
Severe features now include any of these:
systolic blood pressure (BP) of 160 mm Hg or higher, or diastolic BP of 110 mm Hg or higher on two occasions at least 4 hours apart while the patient is on bed rest (unless antihypertensive therapy is initiated before this time)
thrombocytopenia (platelets <100 x 109/L)
impaired liver function, as indicated by abnormally elevated blood concentrations of liver enzymes (to twice normal concentration) and/or severe, persistent right upper quadrant or epigastric pain unresponsive to medication and not accounted for by alternative diagnoses
progressive renal insufficiency (serum creatinine concentration >1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease)
pulmonary edema
new-onset visual or central nervous system disturbances.
Related Article: Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia? George Macones, MD (Examining the Evidence, December 2012)
Screening for preeclampsia. The use of Doppler studies and serum biomarkers is not recommended, as there is no evidence that early identification translates to improved outcomes.
Prevention of preeclampsia. Low-dose aspirin (60–80 mg/d, starting in the late first trimester) should be offered as primary prevention to:
women with a history of early-onset preeclampsia and delivery before 34 weeks’ gestation
women with a history of preeclampsia in multiple pregnancies
other high-risk patients (chronic hypertension, diabetes).
No other treatments (vitamin C or E, salt restriction, or bed rest) are recommended for the prevention of preeclampsia, although calcium supplementation may be recommended for women with a low baseline dietary intake of calcium.
Use of magnesium sulfate. Universal prophylaxis with magnesium sulfate is not recommended for preeclampsia unless severe features are present or the patient’s clinical condition changes to severe during labor.
Timing of delivery. Recommendations for delivery for patients with hypertensive disorders are:
gestational hypertension or preeclampsia without severe features: 37 weeks’ gestation
preeclampsia with severe features: by 34 weeks
chronic hypertension: not before 38 weeks
chronic hypertension with superimposed preeclampsia: 34 or 37 weeks, depending on the presence of severe features.
All of these recommendations are contingent upon the clinical status of the patient and her fetus. For example, if the fetus develops severe growth restriction (<5%) or oligohydramnios, delivery may be recommended regardless of gestational age, based on fetal testing and maternal stability.
Related Article: A stepwise approach to managing eclampsia and other hypertensive emergencies Baha M. Sibai (October 2013)
Postpartum hypertension. The need for recognition of hypertension in the postpartum period is emphasized, as well as appropriate management, using the following guidelines:
Be aware that BP decreases initially after delivery and then increases 3 to 6 days postpartum, requiring vigilance on the part of the clinician. For this reason, BP monitoring is recommended 72 hours postpartum (inpatient or outpatient) and again in 7 to 10 days in women diagnosed with a hypertensive disorder of pregnancy.
Counsel patients who experience a hypertensive disorder and/or preeclampsia during pregnancy about postpartum preeclampsia, providing strict precautions and explicit instructions regarding its signs and symptoms
If BP remains elevated after the first postpartum day, consider discontinuing nonsteroidal anti-inflammatory drugs (NSAIDs), as they may be related to hypertension
Treat BP that remains above 150/100 mm Hg with antihypertensive therapy
If postpartum preeclampsia is suspected, administer magnesium sulfate for 24 hours.
A culture shift is needed
At our institution, the most significant potential changes to clinical practice may be the elimination of universal magnesium sulfate prophylaxis and the removal of severe fetal growth restriction from the definition of “severe” preeclampsia.
Also, because the use of NSAIDs is widespread for postpartum pain control, a culture change is needed if we are to follow the postpartum recommendations.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although we suggest that you read the ACOG report thoroughly, remember that its recommendations are only that—recommendations. Also keep in mind that only six of the approximately 60 “recommendations” provided in this report were accompanied by both high-quality evidence and a strong recommendation. There still is room for clinical judgment and individualization of management to specific patient populations.
NONINVASIVE PRENATAL SCREENING IS EXPANDING RAPIDLY—BUT DON’T THROW OUT THAT CVS KIT JUST YET!
ACOG Committee on Genetics.Committee Opinion #545: Noninvasive prenatal testing for fetal aneuploidy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120(6):1532–1534.
Mennuti MT, Cherry AM, Morrissette JJD, Dugoff L. Is it time to sound an alarm about false-positive cell-free DNA testing for fetal aneuploidy? Am J Obstet Gynecol. 2013;209(5):415–419.
In last year’s Update in Obstetrics, we discussed noninvasive prenatal genetic screening via cell-free fetal DNA, noting that it is a safer (no risk of miscarriage) and faster (starting at 10 weeks’ gestation) way to screen for aneuploidy. The sensitivity and specificity of this test for Trisomy 21 and 18 are over 99%, with slightly lower sensitivity for Trisomy 13 and sex chromosome abnormalities and a false-positive rate of 0.5%.
In a committee opinion published in December 2012, ACOG concluded that
cell-free fetal DNA is an appropriate screening option only for specific groups of patients at risk for aneuploidy:
women older than age 35
women with fetal ultrasonography findings that are concerning for aneuploidy
women with aneuploidy in a prior pregnancy
women with abnormal first- or second-trimester genetic screening tests
parents with a balanced translocation and an increased risk of Trisomy 21 or 13.
Noninvasive aneuploidy testing is for screening only
Negative results are not diagnostic, and all positive results should be confirmed with invasive testing (chorionic villus sampling [CVS] or amniocentesis).
ACOG does not recommend routine use of cell-free fetal DNA without a comprehensive history and adequate patient counseling, as well as a designation of “high risk.”
Over the past year, more options have become available for aneuploidy screening via cell-free fetal DNA, including screening in twin gestations (for Trisomy 21, 18, 13, and the presence of a Y chromosome only) and screening for:
22q deletion (DiGeorge syndrome)
5p– (Cri-du-chat syndrome)
15q (Prader-Willi and Angelman syndromes)
1p (1p36 deletion syndrome)
Trisomy 16
Trisomy 22.
At this rate, the genetic information potentially available via noninvasive testing seems unlimited. It is easy to see how the fact that this is a screening test—not a diagnostic test—could get lost in the excitement.
Other limitations: Noninvasive testing is still not validated in low-risk patients, and the false-positive risk may be higher than original estimates.
Related Article: Noninvasive prenatal DNA testing: A survey of who is using it, and how (Audiocast, June 2013)
The problem of false positives
This issue was addressed by Mennuti and colleagues, who presented eight cases of abnormal cell-free fetal DNA results that were not confirmed by invasive testing.
There are few prospective data about the source of false-positive results; potential mechanisms include an inadequate fetal fraction of cell-free DNA, maternal or placental mosaicism, and a vanishing twin.
Mennuti and colleagues propose that a registry of false-positive and false-negative results be established to gather further data. They also note that as low-risk patients and aneuploidies of lower and lower prevalence are incorporated into noninvasive testing, the false-positive rate will rise. Their findings have implications for patient counseling, patient distress, invasive testing, and reimbursement.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Cell-free fetal DNA is a rapidly expanding screening technology, but it is not ready to replace diagnostic testing. Don’t throw away those CVS and amniocentesis kits just yet—we are a long way from a completely noninvasive world.
WHEN IT COMES TO GESTATIONAL DIABETES, LESS MAY BE MORE
ACOG Committee on Practice Bulletins–Obstetrics. Practice Bulletin #137: Gestational diabetes mellitus. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122(2 Pt 1):406–416.
Gestational diabetes accounts for 90% of diabetic pregnancies, and its incidence has been increasing in the United States along with the obesity epidemic. In recent years, there has been some debate about the best way to screen for, diagnose, and treat gestational diabetes.
Multiple criteria exist for a positive 1-hour (130–140 mg/dL) or 3-hour glucose tolerance test (Carpenter and Coustan vs National Diabetes Data Group), without comparative trials or consensus as to which version is best. Lower cutoffs increase the rate of gestational diabetes by as much as 50%, whereas higher cutoffs lower the false-positive rate and reduce the need for additional tests.
Some groups have recommended moving away from the traditional two-step process to a one-step approach that utilizes the 2-hour, 75-g glucose tolerance test commonly used outside of pregnancy. They argue that this approach would simplify and standardize the process and could improve outcomes in “borderline” pregnancies that would have been missed by less stringent guidelines.
However, the baseline rate of gestational diabetes using this one-step approach would likely increase from 7% to 18% or higher, depending on the patient population. Such an increase would trigger a huge rise in costs and resources needed to care for these patients, without data on outcomes or appropriate therapy for this expanded group of women with gestational diabetes.
Treatment isn't clear-cut, either
Treatment of gestational diabetes centers on labor-intensive glucose monitoring, nutritional interventions, and insulin therapy.
Until recently, the use of oral hypoglycemic agents was not recommended due to limited data. Multiple studies now have been performed to evaluate the safety and efficacy of glyburide and metformin in pregnancy, demonstrating glucose control similar to that achieved with insulin without short-term adverse effects in the mother or newborn. However, as many as 20% to 40% of women using glyburide and 50% of those using metformin require the addition of insulin for adequate glucose control. The long-term effects of these medications are unknown.
Related Article: Does myo-inositol supplementation reduce the rate of gestational diabetes in pregnant women with a family history of type 2 diabetes? E. Albert Reece, MD, PhD, MBA (Examining the Evidence, June 2013)
ACOG weighs in
In an attempt to clarify optimal screening, diagnosis, and treatment, ACOG updated its practice bulletin on gestational diabetes in August 2013. Among its recommendations:
Avoid the 2-hour glucose tolerance test because there is no demonstrated benefit for the increased number of mothers (and their fetuses) that would be identified by this approach. Rather, use the two-step approach of a 1-hour 50-g glucose tolerance test followed by a 3-hour 100-g glucose tolerance test.
In regard to the 1-hour test, ACOG finds either 135 or 140 mg/dL acceptable as a cutoff but recommends that each practice choose one value as a standard and use it consistently. For the 3-hour test, ACOG recommends that each practice choose the version that best fits its population and prevalence of diabetes. At our institution, for example, we have chosen a 1-hour cutoff of 140 mg/dL and the National Diabetes Data Group criteria for the 3-hour test (105, 190, 165, and 145 mg/dL).
Oral glyburide or metformin may be used to treat gestational diabetes, but glyburide (starting at 2.5 mg/d) may be the better choice for glucose control.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Use the two-step screening process to identify gestational diabetes, picking your cutoffs and sticking to them. Oral hypoglycemic agents now are acceptable for treatment, but be prepared to go back to insulin if necessary.
WE WANT TO HEAR FROM YOU. Tell us what you think.
Reference
Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526.
Reference
Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526.
How I counsel patients regarding delayed cord clamping
Has the evidence tipped in favor of delayed cord clamping?
In December 2012, the American College of Obstetricians and Gynecologists
(ACOG) published a Committee Opinion on the timing of umbilical cord clamping after birth, but it found insufficient evidence to recommend early or delayed clamping.1
In a Cochrane review published earlier this year, McDonald and colleagues reviewed 15 trials and 3,911 mother-infant pairs, exploring the primary outcomes of severe maternal postpartum hemorrhage (≥1,000 mL), maternal death, and severe maternal morbidity and neonatal death associated with early versus delayed clamping. They also analyzed a number of secondary outcomes. None of the primary outcomes reached statistical significance.
In a statement from the World Health Organization (WHO) included in the Cochrane review, it was recommended that “the cord should not be clamped earlier than necessary”; the WHO graded this as a “weak recommendation”
based on “low-quality” evidence.
With such underwhelming evidence, I would guess that the average clinician does not feel very motivated to change his or her practice, if that practice involves early clamping.
One limitation of the Cochrane findings
In the studies included in the Cochrane review, there was marked heterogeneity in the definition of delayed cord clamping, which ranged
from 1 minute after delivery to the complete cessation of cord pulsation (~5 minutes). Some of the studies even used alternate times (2 minutes, 3 minutes, and so on).
Delayed clamping improved neonatal hemoglobin status
Among the secondary outcomes assessed in this review was an improvement in neonatal hemoglobin concentration and overall iron stores associated with delayed clamping—but this benefit came at the expense of a higher incidence of neonatal jaundice requiring phototherapy. As a result, the investigators
concluded that delayed cord clamping should be performed when there is ready
access to phototherapy.
Should we implement delayed clamping?
At this time, I am reluctant to recommend that we shift to delayed clamping. Here are my reasons:
- Data are lacking as to whether increased hemoglobin levels and iron stores in newborns improve outcomes—or provide any benefit. No long-term developmental outcome data were included in the Cochrane review.
- Although I am not a pediatrician, I am unaware of infant iron deficiency being a significant threat to public health in the developed world.
- The greater need for phototherapy in the delayed-clamping group should not be viewed as inconsequential.
- Iron supplementation is probably more readily available than phototherapy, especially in developing countries.
- In the minority of cases in which delayed clamping might be beneficial (eg, prematurity), it is not always feasible, as these infants may already be compromised. Anxious neonatologists generally want the newborn handed over to them for resuscitation as quickly as possible, generally frowning upon a delay of 3 to 5 minutes for the blood to move from the placenta to the infant.
What this evidence means for practice
I recommend that obstetric care providers continue their current practice until more detailed data emerge on the risks and benefits of delayed clamping. If a patient asks about the issue, we should counsel her about the risks and benefits of early versus delayed clamping and comply with her choice when there are no contraindications.
—John T. Repke, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
Reference
1. American College of Obstetricians and Gynecologists. Committee Opinion #543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012:120(6):1522–1526.
In December 2012, the American College of Obstetricians and Gynecologists
(ACOG) published a Committee Opinion on the timing of umbilical cord clamping after birth, but it found insufficient evidence to recommend early or delayed clamping.1
In a Cochrane review published earlier this year, McDonald and colleagues reviewed 15 trials and 3,911 mother-infant pairs, exploring the primary outcomes of severe maternal postpartum hemorrhage (≥1,000 mL), maternal death, and severe maternal morbidity and neonatal death associated with early versus delayed clamping. They also analyzed a number of secondary outcomes. None of the primary outcomes reached statistical significance.
In a statement from the World Health Organization (WHO) included in the Cochrane review, it was recommended that “the cord should not be clamped earlier than necessary”; the WHO graded this as a “weak recommendation”
based on “low-quality” evidence.
With such underwhelming evidence, I would guess that the average clinician does not feel very motivated to change his or her practice, if that practice involves early clamping.
One limitation of the Cochrane findings
In the studies included in the Cochrane review, there was marked heterogeneity in the definition of delayed cord clamping, which ranged
from 1 minute after delivery to the complete cessation of cord pulsation (~5 minutes). Some of the studies even used alternate times (2 minutes, 3 minutes, and so on).
Delayed clamping improved neonatal hemoglobin status
Among the secondary outcomes assessed in this review was an improvement in neonatal hemoglobin concentration and overall iron stores associated with delayed clamping—but this benefit came at the expense of a higher incidence of neonatal jaundice requiring phototherapy. As a result, the investigators
concluded that delayed cord clamping should be performed when there is ready
access to phototherapy.
Should we implement delayed clamping?
At this time, I am reluctant to recommend that we shift to delayed clamping. Here are my reasons:
- Data are lacking as to whether increased hemoglobin levels and iron stores in newborns improve outcomes—or provide any benefit. No long-term developmental outcome data were included in the Cochrane review.
- Although I am not a pediatrician, I am unaware of infant iron deficiency being a significant threat to public health in the developed world.
- The greater need for phototherapy in the delayed-clamping group should not be viewed as inconsequential.
- Iron supplementation is probably more readily available than phototherapy, especially in developing countries.
- In the minority of cases in which delayed clamping might be beneficial (eg, prematurity), it is not always feasible, as these infants may already be compromised. Anxious neonatologists generally want the newborn handed over to them for resuscitation as quickly as possible, generally frowning upon a delay of 3 to 5 minutes for the blood to move from the placenta to the infant.
What this evidence means for practice
I recommend that obstetric care providers continue their current practice until more detailed data emerge on the risks and benefits of delayed clamping. If a patient asks about the issue, we should counsel her about the risks and benefits of early versus delayed clamping and comply with her choice when there are no contraindications.
—John T. Repke, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
In December 2012, the American College of Obstetricians and Gynecologists
(ACOG) published a Committee Opinion on the timing of umbilical cord clamping after birth, but it found insufficient evidence to recommend early or delayed clamping.1
In a Cochrane review published earlier this year, McDonald and colleagues reviewed 15 trials and 3,911 mother-infant pairs, exploring the primary outcomes of severe maternal postpartum hemorrhage (≥1,000 mL), maternal death, and severe maternal morbidity and neonatal death associated with early versus delayed clamping. They also analyzed a number of secondary outcomes. None of the primary outcomes reached statistical significance.
In a statement from the World Health Organization (WHO) included in the Cochrane review, it was recommended that “the cord should not be clamped earlier than necessary”; the WHO graded this as a “weak recommendation”
based on “low-quality” evidence.
With such underwhelming evidence, I would guess that the average clinician does not feel very motivated to change his or her practice, if that practice involves early clamping.
One limitation of the Cochrane findings
In the studies included in the Cochrane review, there was marked heterogeneity in the definition of delayed cord clamping, which ranged
from 1 minute after delivery to the complete cessation of cord pulsation (~5 minutes). Some of the studies even used alternate times (2 minutes, 3 minutes, and so on).
Delayed clamping improved neonatal hemoglobin status
Among the secondary outcomes assessed in this review was an improvement in neonatal hemoglobin concentration and overall iron stores associated with delayed clamping—but this benefit came at the expense of a higher incidence of neonatal jaundice requiring phototherapy. As a result, the investigators
concluded that delayed cord clamping should be performed when there is ready
access to phototherapy.
Should we implement delayed clamping?
At this time, I am reluctant to recommend that we shift to delayed clamping. Here are my reasons:
- Data are lacking as to whether increased hemoglobin levels and iron stores in newborns improve outcomes—or provide any benefit. No long-term developmental outcome data were included in the Cochrane review.
- Although I am not a pediatrician, I am unaware of infant iron deficiency being a significant threat to public health in the developed world.
- The greater need for phototherapy in the delayed-clamping group should not be viewed as inconsequential.
- Iron supplementation is probably more readily available than phototherapy, especially in developing countries.
- In the minority of cases in which delayed clamping might be beneficial (eg, prematurity), it is not always feasible, as these infants may already be compromised. Anxious neonatologists generally want the newborn handed over to them for resuscitation as quickly as possible, generally frowning upon a delay of 3 to 5 minutes for the blood to move from the placenta to the infant.
What this evidence means for practice
I recommend that obstetric care providers continue their current practice until more detailed data emerge on the risks and benefits of delayed clamping. If a patient asks about the issue, we should counsel her about the risks and benefits of early versus delayed clamping and comply with her choice when there are no contraindications.
—John T. Repke, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
Reference
1. American College of Obstetricians and Gynecologists. Committee Opinion #543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012:120(6):1522–1526.
Reference
1. American College of Obstetricians and Gynecologists. Committee Opinion #543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012:120(6):1522–1526.
Which tocolytic agents are most likely to delay delivery and improve neonatal outcomes?
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
This statistical tour de force on the topic of tocolytic therapy attempts to determine which agents are most effective at delaying delivery. The analysis is not a typical meta-analysis but what the investigators refer to as “network meta-analysis,” the distinction being that the latter makes an assumption of consistency because all relevant data are compared, including treatments that may no longer appear to be effective or relevant. This distinction may be a bit too esoteric for most clinicians, me included.
Details of the trial
Investigators identified 95 randomized, controlled trials of tocolytic therapy.
The probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17–5.91), beta mimetics (OR, 2.41; 95% CI, 1.27–4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo.
No class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome.
Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo.
Prostaglandin inhibitors and calcium channel blockers were the agents most likely to be ranked in the top three classes of medication for the outcomes of a 48-hour delay in delivery, lower risk of respiratory distress syndrome and neonatal mortality, and fewer maternal side effects.
Strengths and limitations
The major strength of this analysis is its inclusion of trials from the world literature.
Weaknesses include the:
- lack of practice standardization in many trials
- inclusion of many trials that used composite or secondary analysis
- inability to control for the inclusion of magnesium sulfate in more recent trials, when it was classified as a neuroprotective agent and not a tocolytic
- inclusion of studies involving drugs unavailable in the United States.
The investigators also made some decisions about which trials to include and exclude that could have confused the picture. For example, they excluded trials that used combination drug therapy for tocolysis. As a result, the use of magnesium sulfate as a neuroprotective agent could have confounded the results if it was not recorded as a tocolytic when used in conjunction with another tocolytic.
The most recent practice bulletin from the American College of Obstetricians and Gynecologists addresses this point when it cautions practitioners about developing guidelines for concurrent tocolytic use when employing magnesium sulfate for fetal neuroprotection.1 The practice bulletin also confirms Level A evidence for the use of beta-agonists, calcium channel blockers, or nonsteroidal anti-inflammatory drugs for short-term tocolysis, effectively summarizing current clinical practice and agreeing with the conclusions of Haas and colleagues.
Overall, Haas and colleagues affirm current practice, concluding that prostaglandin inhibitors (eg, indomethacin) and calcium channel blockers (eg, nifedipine, nicardipine) delay delivery and improve short-term neonatal outcomes, whereas beta-agonist therapy was not considered to be as preferable, largely due to its maternal side effect profile. In addition, it is difficult to decipher the relative contributions of the drugs described in this investigation to neonatal and maternal side effects.
I plan to continue my current practice of employing indomethacin or nifedipine as first-line, short-term tocolytic therapy in conjunction with administration of magnesium sulfate for fetal neuroprotection, when appropriate.
I give a starting dose of indomethacin of 50 to 100 mg (orally or rectally), with a maintenance dose of 25 mg orally every 4 hours or 50 mg every 6 hours, not to exceed 48 consecutive hours of treatment.
For nifedipine, I give 20 to 30 mg orally, which can be repeated in 2 hours, if necessary. The maintenance dose is 10 to 20 mg orally every 4 to 6 hours, not to exceed 180 mg/day or 72 consecutive hours of treatment.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
ON OBSTETRICS?
Which skin closure technique better reduces the risk of cesarean wound complications: surgical staples or subcuticular suture?
Dhanya Mackeen, MD, MPH; Vincenzo Berghella, MD (February 2013)
Does an unfavorable cervix preclude induction of labor at term in women who have gestational hypertension or mild preeclampsia?
George Macones, MD (December 2012)
Is the rate of progress the same for induced and spontaneous labors?
William F. Rayburn, MD (November 2012)
Does maternal exposure to magnesium sulfate affect fetal
heart-rate patterns?
John M. Thorp, Jr, MD (October 2012)
Is elective delivery at 37 weeks’ gestation safe in uncomplicated
twin pregnancies?
Steven T. Chasen, MD (September 2012)
Does mediolateral episiotomy reduce the risk of anal sphincter injury in operative vaginal delivery?
Errol T. Norwitz, MD, PhD (August 2012)
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (April 2012)
Does weekly progesterone prolong gestation in women who
have PPROM?
Alex C. Vidaeff, MD, MPH (May 2011)
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.
Reference
1. Committee on Practice Bulletin–Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1307-1317.
UPDATE ON OBSTETRICS
The authors report no financial relationships relevant to this article.
If there have been overriding themes in obstetrics over the past year, they have been “more,” “sooner,” “faster,” “safer.” Advances in our field have thrilled our scientific curiosity and increased our ability to alleviate suffering—but at what cost? And who will pay that cost?
In this Update, we focus on recent advances in prenatal diagnosis and fetal therapy, as well as the ever-encroaching economic barriers that may limit our ability to get what we want. In particular, we will discuss:
- two technologies in prenatal genetics: noninvasive aneuploidy testing using cell-free DNA and prenatal microarray analysis
- open fetal surgery to reduce mortality and improve the function and quality of life for fetuses with open neural tube defects
- the value and probable impact of bundled payments—that is, one payment for multiple services grouped into one “episode.”
Two noninvasive approaches to prenatal diagnosis offer promise—but practicality and cost are uncertain
Ashoor G, Syngelaki RM, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367(23):2185–2193.
Talkowski ME, Ordulu Z, Pillalamarri V, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med. 2012;367(23):2226–2232.
Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
Genetic screening and testing are a standard part of prenatal care in most developed countries. We have come a long way since a maternal age of 35 years was the only variable separating patients into low- and high-risk categories. This year, two technologies have emerged that may change forever the way we approach prenatal genetics:
One argument for using more accurate genetic screening methods: They limit the number of invasive tests that are needed. Chorionic villus sampling (CVS) and amniocentesis, even when performed by the most experienced of operators, pose a small but real risk of fetal injury and pregnancy loss.
Noninvasive aneuploidy diagnosis is now a reality in high-risk population screening
The holy grail of aneuploidy diagnosis would be a noninvasive way to sample fetal cells. Although we have known for decades that fetal cells enter the maternal circulation, it has been impractical to use them for aneuploidy testing because of their scarcity and longevity. In the 1990s, however, cell-free fetal DNA (cffDNA), a compound of DNA fragments of uncertain origin, was identified in maternal plasma. CffDNA is more plentiful than fetal cells. It also disappears within hours of delivery, demonstrating that it is specific to the current pregnancy.
CffDNA is already used in fetal Rh typing and gender determination in disorders such as congenital adrenal hyperplasia. Several studies in high-risk populations have demonstrated high sensitivity and specificity for the detection of Trisomies 21, 18, and 13. Several commercial tests are now available, although neither their accuracy nor their cost has been determined for use in low-risk population screening, compared with traditional testing.
Microarray analysis, paired with karyotyping, can elucidate ultrasound-identified fetal anomalies
Cytogenetic microarray analysis is also being explored in the prenatal period. Microarray analysis is currently used as a first-line test for infants and children who demonstrate developmental delay, autism spectrum disorders, dysmorphic features, and congenital anomalies. As many as 15% of patients with an otherwise normal karyotype will have a clinically significant copy number variant (CNV) on microarray. This finding has led to the use of microarray analysis in conjunction with karyotyping for fetuses with ultrasound-identified anomalies. Both targeted arrays (for syndromes associated with ultrasound anomalies) and whole-genome arrays are available.
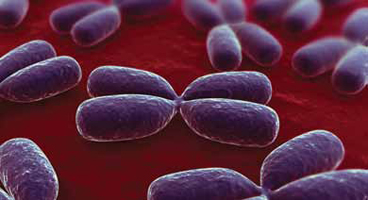
Recent data from a study from the National Institute of Child Health and Human Development (NICHD) reveal that the prenatal detection rates for aneuploidy and unbalanced translocations are comparable between microarray analysis and karyotyping. Microarray analysis did not, however, detect triploidies or balanced translocations. As many as 6% of patients with a normal karyotype and structural anomalies and 1.7% of patients with advanced maternal age or positive screening tests had either a known or potentially clinically relevant CNV. This large study concluded that microarray analysis not only provides equal detection of aneuploidy but also more information in the form of CNVs, compared with karyotyping alone.
Microarray analysis also has been used in the study of pregnancy loss and stillbirth because it does not require viable or intact tissue as a source of DNA—an advantage, compared with traditional karyotyping. A recent study from the Stillbirth Collaborative Research Network demonstrated that genetic results in cases involving stillbirth were obtained more frequently via microarray analysis (87.4%) than by karyotype (70.5%). In addition, more genetic abnormalities (aneuploidy, pathogenic CNVs, and CNVs of unknown clinical significance) were detected by microarray analysis. Investigators concluded that microarray analysis may be especially useful in cases involving stillbirth (when a karyotype cannot be obtained) and structural abnormalities.
We want an accurate, completely risk-free genetic test that can be used for anyone. What we have so far is a technology that must be tested before it can be used in most of our patients—that is, the low-risk ones. We also have access to the fetus’ genetic code on a very specific level.
The total costs of such an approach—test, interpretation, counseling, and long-term follow-up of uncertain results—are unknown at this time and may prove to be unaffordable on a population-wide basis.
Should microarray analysis replace routine prenatal genetic testing?
A major dilemma associated with this technology is the significant amount of time that may be needed to counsel patients when the results are of unclear clinical significance.5 If the fetus has an anomaly, and a related CNV is identified, then counseling of the parents is fairly straightforward. However, if the fetus has an anomaly and a CNV that has not yet been defined, what should the parents be told? Some argue that this information should not be shared with the parents, whereas others recommend full disclosure of all results—even if we do not yet know what to make of them.
Another issue with microarray analysis is its inability to detect balanced translocations, triploidies, and low-level mosaicism, which require either a karyotype or whole-genome sequencing. Microarray analysis is also more expensive than karyotyping, although this may change in the future.
>Fetal therapy involves a complex equation of potential benefits and risks
Adzick NS, Thom EA, Spong CY, et al; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1104.
Fetal therapy is broadly defined as any intervention administered to or via the mother with a primary indication to improve perinatal or long-term outcomes for the fetus or newborn. The concept of intervening to prevent the death of a fetus by correcting an anatomic anomaly or halting a disease process in utero is not new. Liley performed the first intrauterine fetal transfusion for Rh alloimmunization in the 1960s. Today, we perform fetal interventions routinely to reduce mortality by giving medical therapy to the mother, such as antenatal corticosteroids to enhance fetal lung maturity or anti-arrhythmics for supraventricular tachycardia. More invasive procedures have proved to be lifesaving (placental laser coagulation for twin-twin transfusion syndrome), ameliorating in the short term (shunting for lower urinary tract obstruction to relieve oligohydramnios), or ultimately not helpful (decompression of hydrocephalus).
Most recently, open fetal surgery has taken center stage as an intervention focused not only on reducing mortality but on improving function and quality of life for fetuses with open neural tube defects (ONTDs). This anomaly was targeted for fetal intervention because, although ONTDs are not generally considered lethal, a significant number of patients die before the age of 5, the majority of patients require shunts that leave them vulnerable to complications, and ONTDs generally impose lifelong intellectual and physical limitations. Repair during fetal life was proposed to prevent damage to the spinal cord and reverse hindbrain herniation, with the goal of improving long-term neurologic function.
The Management of Myelomeningocele Study (MOMS) is a prospective, multicenter trial that randomly assigned fetuses with isolated ONTDs to open fetal repair of myelomeningocele via hysterotomy or to postnatal repair of the defect. Forty percent of infants who underwent fetal repair required placement of a shunt, compared with 82% of those who had postnatal repair (relative risk, 0.48; 97.7% CI, 0.36 to 0.64; P<.001). Infants in the fetal-repair group also had significantly improved composite scores for mental development and motor function at 30 months (P = .007), as well as improvement in secondary outcomes such as hindbrain herniation and independent walking at 30 months.
As exciting as these results are, open fetal surgery still has significant limitations. The few centers that perform the most complex surgeries often have strict exclusion criteria, including maternal body mass index (BMI) greater than 35 kg/m2 and other medical comorbidities. The surgery also poses real risks for both mother and fetus. In the MOMS trial, the risk of preterm labor increased in the fetal-repair group, compared with postnatal repair (38% vs 14%), as did the risk of premature rupture of membranes (46% vs 8%). The fetal-repair group delivered more than 3 weeks earlier than the postnatal repair group (34 vs 37 weeks). Twenty-five percent of the fetal-repair group had thinning of the uterine scar, with uterine dehiscence seen in 10%. When myelomeningocele is repaired during fetal life, mothers require two hysterotomies during pregnancy and face an increased risk of uterine rupture and preterm delivery in subsequent pregnancies. The use of tocolytics exposes these mothers to an increased risk of pulmonary edema (6% in the fetal-repair group vs 0% for postnatal repair).
Other issues that should be addressed:
- the need for rigorous study of open fetal surgery for other fetal anomalies
- prognostic factors for success and for complications
- long-term outcomes in neurologic development of children and fertility of mothers
- a comparison of costs between fetal and postnatal treatment.
Although we may want to intervene as early in life as possible (that is, the fetal period) to achieve the best outcomes for the child, we need to weigh the short-term benefits of intervention against the known risks that intervention poses for the mother in the current pregnancy as well as the potential implications for future pregnancies (ie, the need for all future deliveries to be by cesarean section), not to mention the unknown long-term effects of intervention on both the child and society.
Bundled payments may help us deliver higher-quality, more efficient, and less costly care
Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Fact Sheet: Bundled Payments for Care Improvement Initiative. Washington, DC: DHS; 2011. http://innovations.cms.gov/Files/fact-sheet/Bundled-Payment-Fact-Sheet.pdf. Accessed December 6, 2012.
There is little question that health care in the United States needs reform. The culture of “more is better” is not sustainable economically—nor does our health as a whole reflect the amount of money that we spend on health care, compared with other countries. Although the future is not yet clear, one proposed mechanism for reform is the institution of bundled payments—the grouping of multiple services into one “episode” for payment purposes. An episode might include inpatient hospitalization for pneumonia, for example, or the grouping of surgery with post-discharge care. In obstetrics, all pregnancy care could be grouped into one episode. The concept behind bundled payments is to provide incentives to institutions and providers to delivery higher-quality, more efficient, and less costly care.
If bundled payments become the reality for obstetric care in the future, how will that affect the way we care for our patients? Instead of blindly ordering all available tests, we need to consider thoroughly whether the patient truly needs a test to improve pregnancy outcomes. We also need to consider whether other measures might be avoided safely to keep costs within the bundle. A few examples:
- Is a screening fetal echocardiogram really necessary in a diabetic woman if the ultrasound anatomy scan is sufficient to rule out any cardiac anomaly that might require intervention in the delivery room?
- How will we integrate the expense of cell-free fetal DNA aneuploidy testing and microarray analysis, not to mention the extended counseling sessions that will be necessary to explain findings of uncertain clinical significance, into the bundle? Will “low-risk” patients need to pay out of pocket?
Few physicians entered medicine to worry about costs. Most of us want to worry about our patients. Yet, the reality is that scientific curiosity and a desire to do more—and to do it sooner, faster, and safer—are no longer sufficient justifications for many clinical decisions. We soon may need to figure out how to get what we need without spending as much in the process. In doing so, we may find ourselves moving away from the computer screen and back to the bedside—where we belong.
- Will a second ultrasound scan to visualize the fetal spine in a patient with a normal alpha-fetoprotein level be included in the bundle or paid for by the patient?
These issues may seem trivial, but we can no longer afford to order every test available. We will need to spend more time examining and counseling our patients so that they feel they are still getting the best care possible.
We want to hear from you! Tell us what you think.
1. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis by fetal nucleic acid analysis in maternal plasma: the coming of age. Semin Fetal Neonatal Med. 2011;16(2):88-93.
2. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis empowered by high-throughput sequencing. Prenat Diagn. 2012;32(4):401-406.
3. Savage MS, Mourad MJ, Wapner RJ. Evolving applications of microarray analysis in prenatal diagnosis. Curr Opin Obstet Gynecol. 2011;23(2):103-108.
4. Dugoff L. Application of genomic technology in prenatal diagnosis [editorial]. N Engl J Med. 2012;367(23):2249-2251.
5. Wapner RJ, Driscoll DA, Simpson JL. Integration of microarray technology into prenatal diagnosis: counseling issues generated during the NICHD clinical trial. Prenat Diagn. 2012;32(4):396-400.
The authors report no financial relationships relevant to this article.
If there have been overriding themes in obstetrics over the past year, they have been “more,” “sooner,” “faster,” “safer.” Advances in our field have thrilled our scientific curiosity and increased our ability to alleviate suffering—but at what cost? And who will pay that cost?
In this Update, we focus on recent advances in prenatal diagnosis and fetal therapy, as well as the ever-encroaching economic barriers that may limit our ability to get what we want. In particular, we will discuss:
- two technologies in prenatal genetics: noninvasive aneuploidy testing using cell-free DNA and prenatal microarray analysis
- open fetal surgery to reduce mortality and improve the function and quality of life for fetuses with open neural tube defects
- the value and probable impact of bundled payments—that is, one payment for multiple services grouped into one “episode.”
Two noninvasive approaches to prenatal diagnosis offer promise—but practicality and cost are uncertain
Ashoor G, Syngelaki RM, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367(23):2185–2193.
Talkowski ME, Ordulu Z, Pillalamarri V, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med. 2012;367(23):2226–2232.
Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
Genetic screening and testing are a standard part of prenatal care in most developed countries. We have come a long way since a maternal age of 35 years was the only variable separating patients into low- and high-risk categories. This year, two technologies have emerged that may change forever the way we approach prenatal genetics:
One argument for using more accurate genetic screening methods: They limit the number of invasive tests that are needed. Chorionic villus sampling (CVS) and amniocentesis, even when performed by the most experienced of operators, pose a small but real risk of fetal injury and pregnancy loss.
Noninvasive aneuploidy diagnosis is now a reality in high-risk population screening
The holy grail of aneuploidy diagnosis would be a noninvasive way to sample fetal cells. Although we have known for decades that fetal cells enter the maternal circulation, it has been impractical to use them for aneuploidy testing because of their scarcity and longevity. In the 1990s, however, cell-free fetal DNA (cffDNA), a compound of DNA fragments of uncertain origin, was identified in maternal plasma. CffDNA is more plentiful than fetal cells. It also disappears within hours of delivery, demonstrating that it is specific to the current pregnancy.
CffDNA is already used in fetal Rh typing and gender determination in disorders such as congenital adrenal hyperplasia. Several studies in high-risk populations have demonstrated high sensitivity and specificity for the detection of Trisomies 21, 18, and 13. Several commercial tests are now available, although neither their accuracy nor their cost has been determined for use in low-risk population screening, compared with traditional testing.
Microarray analysis, paired with karyotyping, can elucidate ultrasound-identified fetal anomalies
Cytogenetic microarray analysis is also being explored in the prenatal period. Microarray analysis is currently used as a first-line test for infants and children who demonstrate developmental delay, autism spectrum disorders, dysmorphic features, and congenital anomalies. As many as 15% of patients with an otherwise normal karyotype will have a clinically significant copy number variant (CNV) on microarray. This finding has led to the use of microarray analysis in conjunction with karyotyping for fetuses with ultrasound-identified anomalies. Both targeted arrays (for syndromes associated with ultrasound anomalies) and whole-genome arrays are available.

Recent data from a study from the National Institute of Child Health and Human Development (NICHD) reveal that the prenatal detection rates for aneuploidy and unbalanced translocations are comparable between microarray analysis and karyotyping. Microarray analysis did not, however, detect triploidies or balanced translocations. As many as 6% of patients with a normal karyotype and structural anomalies and 1.7% of patients with advanced maternal age or positive screening tests had either a known or potentially clinically relevant CNV. This large study concluded that microarray analysis not only provides equal detection of aneuploidy but also more information in the form of CNVs, compared with karyotyping alone.
Microarray analysis also has been used in the study of pregnancy loss and stillbirth because it does not require viable or intact tissue as a source of DNA—an advantage, compared with traditional karyotyping. A recent study from the Stillbirth Collaborative Research Network demonstrated that genetic results in cases involving stillbirth were obtained more frequently via microarray analysis (87.4%) than by karyotype (70.5%). In addition, more genetic abnormalities (aneuploidy, pathogenic CNVs, and CNVs of unknown clinical significance) were detected by microarray analysis. Investigators concluded that microarray analysis may be especially useful in cases involving stillbirth (when a karyotype cannot be obtained) and structural abnormalities.
We want an accurate, completely risk-free genetic test that can be used for anyone. What we have so far is a technology that must be tested before it can be used in most of our patients—that is, the low-risk ones. We also have access to the fetus’ genetic code on a very specific level.
The total costs of such an approach—test, interpretation, counseling, and long-term follow-up of uncertain results—are unknown at this time and may prove to be unaffordable on a population-wide basis.
Should microarray analysis replace routine prenatal genetic testing?
A major dilemma associated with this technology is the significant amount of time that may be needed to counsel patients when the results are of unclear clinical significance.5 If the fetus has an anomaly, and a related CNV is identified, then counseling of the parents is fairly straightforward. However, if the fetus has an anomaly and a CNV that has not yet been defined, what should the parents be told? Some argue that this information should not be shared with the parents, whereas others recommend full disclosure of all results—even if we do not yet know what to make of them.
Another issue with microarray analysis is its inability to detect balanced translocations, triploidies, and low-level mosaicism, which require either a karyotype or whole-genome sequencing. Microarray analysis is also more expensive than karyotyping, although this may change in the future.
>Fetal therapy involves a complex equation of potential benefits and risks
Adzick NS, Thom EA, Spong CY, et al; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1104.
Fetal therapy is broadly defined as any intervention administered to or via the mother with a primary indication to improve perinatal or long-term outcomes for the fetus or newborn. The concept of intervening to prevent the death of a fetus by correcting an anatomic anomaly or halting a disease process in utero is not new. Liley performed the first intrauterine fetal transfusion for Rh alloimmunization in the 1960s. Today, we perform fetal interventions routinely to reduce mortality by giving medical therapy to the mother, such as antenatal corticosteroids to enhance fetal lung maturity or anti-arrhythmics for supraventricular tachycardia. More invasive procedures have proved to be lifesaving (placental laser coagulation for twin-twin transfusion syndrome), ameliorating in the short term (shunting for lower urinary tract obstruction to relieve oligohydramnios), or ultimately not helpful (decompression of hydrocephalus).
Most recently, open fetal surgery has taken center stage as an intervention focused not only on reducing mortality but on improving function and quality of life for fetuses with open neural tube defects (ONTDs). This anomaly was targeted for fetal intervention because, although ONTDs are not generally considered lethal, a significant number of patients die before the age of 5, the majority of patients require shunts that leave them vulnerable to complications, and ONTDs generally impose lifelong intellectual and physical limitations. Repair during fetal life was proposed to prevent damage to the spinal cord and reverse hindbrain herniation, with the goal of improving long-term neurologic function.
The Management of Myelomeningocele Study (MOMS) is a prospective, multicenter trial that randomly assigned fetuses with isolated ONTDs to open fetal repair of myelomeningocele via hysterotomy or to postnatal repair of the defect. Forty percent of infants who underwent fetal repair required placement of a shunt, compared with 82% of those who had postnatal repair (relative risk, 0.48; 97.7% CI, 0.36 to 0.64; P<.001). Infants in the fetal-repair group also had significantly improved composite scores for mental development and motor function at 30 months (P = .007), as well as improvement in secondary outcomes such as hindbrain herniation and independent walking at 30 months.
As exciting as these results are, open fetal surgery still has significant limitations. The few centers that perform the most complex surgeries often have strict exclusion criteria, including maternal body mass index (BMI) greater than 35 kg/m2 and other medical comorbidities. The surgery also poses real risks for both mother and fetus. In the MOMS trial, the risk of preterm labor increased in the fetal-repair group, compared with postnatal repair (38% vs 14%), as did the risk of premature rupture of membranes (46% vs 8%). The fetal-repair group delivered more than 3 weeks earlier than the postnatal repair group (34 vs 37 weeks). Twenty-five percent of the fetal-repair group had thinning of the uterine scar, with uterine dehiscence seen in 10%. When myelomeningocele is repaired during fetal life, mothers require two hysterotomies during pregnancy and face an increased risk of uterine rupture and preterm delivery in subsequent pregnancies. The use of tocolytics exposes these mothers to an increased risk of pulmonary edema (6% in the fetal-repair group vs 0% for postnatal repair).
Other issues that should be addressed:
- the need for rigorous study of open fetal surgery for other fetal anomalies
- prognostic factors for success and for complications
- long-term outcomes in neurologic development of children and fertility of mothers
- a comparison of costs between fetal and postnatal treatment.
Although we may want to intervene as early in life as possible (that is, the fetal period) to achieve the best outcomes for the child, we need to weigh the short-term benefits of intervention against the known risks that intervention poses for the mother in the current pregnancy as well as the potential implications for future pregnancies (ie, the need for all future deliveries to be by cesarean section), not to mention the unknown long-term effects of intervention on both the child and society.
Bundled payments may help us deliver higher-quality, more efficient, and less costly care
Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Fact Sheet: Bundled Payments for Care Improvement Initiative. Washington, DC: DHS; 2011. http://innovations.cms.gov/Files/fact-sheet/Bundled-Payment-Fact-Sheet.pdf. Accessed December 6, 2012.
There is little question that health care in the United States needs reform. The culture of “more is better” is not sustainable economically—nor does our health as a whole reflect the amount of money that we spend on health care, compared with other countries. Although the future is not yet clear, one proposed mechanism for reform is the institution of bundled payments—the grouping of multiple services into one “episode” for payment purposes. An episode might include inpatient hospitalization for pneumonia, for example, or the grouping of surgery with post-discharge care. In obstetrics, all pregnancy care could be grouped into one episode. The concept behind bundled payments is to provide incentives to institutions and providers to delivery higher-quality, more efficient, and less costly care.
If bundled payments become the reality for obstetric care in the future, how will that affect the way we care for our patients? Instead of blindly ordering all available tests, we need to consider thoroughly whether the patient truly needs a test to improve pregnancy outcomes. We also need to consider whether other measures might be avoided safely to keep costs within the bundle. A few examples:
- Is a screening fetal echocardiogram really necessary in a diabetic woman if the ultrasound anatomy scan is sufficient to rule out any cardiac anomaly that might require intervention in the delivery room?
- How will we integrate the expense of cell-free fetal DNA aneuploidy testing and microarray analysis, not to mention the extended counseling sessions that will be necessary to explain findings of uncertain clinical significance, into the bundle? Will “low-risk” patients need to pay out of pocket?
Few physicians entered medicine to worry about costs. Most of us want to worry about our patients. Yet, the reality is that scientific curiosity and a desire to do more—and to do it sooner, faster, and safer—are no longer sufficient justifications for many clinical decisions. We soon may need to figure out how to get what we need without spending as much in the process. In doing so, we may find ourselves moving away from the computer screen and back to the bedside—where we belong.
- Will a second ultrasound scan to visualize the fetal spine in a patient with a normal alpha-fetoprotein level be included in the bundle or paid for by the patient?
These issues may seem trivial, but we can no longer afford to order every test available. We will need to spend more time examining and counseling our patients so that they feel they are still getting the best care possible.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
If there have been overriding themes in obstetrics over the past year, they have been “more,” “sooner,” “faster,” “safer.” Advances in our field have thrilled our scientific curiosity and increased our ability to alleviate suffering—but at what cost? And who will pay that cost?
In this Update, we focus on recent advances in prenatal diagnosis and fetal therapy, as well as the ever-encroaching economic barriers that may limit our ability to get what we want. In particular, we will discuss:
- two technologies in prenatal genetics: noninvasive aneuploidy testing using cell-free DNA and prenatal microarray analysis
- open fetal surgery to reduce mortality and improve the function and quality of life for fetuses with open neural tube defects
- the value and probable impact of bundled payments—that is, one payment for multiple services grouped into one “episode.”
Two noninvasive approaches to prenatal diagnosis offer promise—but practicality and cost are uncertain
Ashoor G, Syngelaki RM, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206(4):322.e1–e5.
Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367(23):2185–2193.
Talkowski ME, Ordulu Z, Pillalamarri V, et al. Clinical diagnosis by whole-genome sequencing of a prenatal sample. N Engl J Med. 2012;367(23):2226–2232.
Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
Genetic screening and testing are a standard part of prenatal care in most developed countries. We have come a long way since a maternal age of 35 years was the only variable separating patients into low- and high-risk categories. This year, two technologies have emerged that may change forever the way we approach prenatal genetics:
One argument for using more accurate genetic screening methods: They limit the number of invasive tests that are needed. Chorionic villus sampling (CVS) and amniocentesis, even when performed by the most experienced of operators, pose a small but real risk of fetal injury and pregnancy loss.
Noninvasive aneuploidy diagnosis is now a reality in high-risk population screening
The holy grail of aneuploidy diagnosis would be a noninvasive way to sample fetal cells. Although we have known for decades that fetal cells enter the maternal circulation, it has been impractical to use them for aneuploidy testing because of their scarcity and longevity. In the 1990s, however, cell-free fetal DNA (cffDNA), a compound of DNA fragments of uncertain origin, was identified in maternal plasma. CffDNA is more plentiful than fetal cells. It also disappears within hours of delivery, demonstrating that it is specific to the current pregnancy.
CffDNA is already used in fetal Rh typing and gender determination in disorders such as congenital adrenal hyperplasia. Several studies in high-risk populations have demonstrated high sensitivity and specificity for the detection of Trisomies 21, 18, and 13. Several commercial tests are now available, although neither their accuracy nor their cost has been determined for use in low-risk population screening, compared with traditional testing.
Microarray analysis, paired with karyotyping, can elucidate ultrasound-identified fetal anomalies
Cytogenetic microarray analysis is also being explored in the prenatal period. Microarray analysis is currently used as a first-line test for infants and children who demonstrate developmental delay, autism spectrum disorders, dysmorphic features, and congenital anomalies. As many as 15% of patients with an otherwise normal karyotype will have a clinically significant copy number variant (CNV) on microarray. This finding has led to the use of microarray analysis in conjunction with karyotyping for fetuses with ultrasound-identified anomalies. Both targeted arrays (for syndromes associated with ultrasound anomalies) and whole-genome arrays are available.

Recent data from a study from the National Institute of Child Health and Human Development (NICHD) reveal that the prenatal detection rates for aneuploidy and unbalanced translocations are comparable between microarray analysis and karyotyping. Microarray analysis did not, however, detect triploidies or balanced translocations. As many as 6% of patients with a normal karyotype and structural anomalies and 1.7% of patients with advanced maternal age or positive screening tests had either a known or potentially clinically relevant CNV. This large study concluded that microarray analysis not only provides equal detection of aneuploidy but also more information in the form of CNVs, compared with karyotyping alone.
Microarray analysis also has been used in the study of pregnancy loss and stillbirth because it does not require viable or intact tissue as a source of DNA—an advantage, compared with traditional karyotyping. A recent study from the Stillbirth Collaborative Research Network demonstrated that genetic results in cases involving stillbirth were obtained more frequently via microarray analysis (87.4%) than by karyotype (70.5%). In addition, more genetic abnormalities (aneuploidy, pathogenic CNVs, and CNVs of unknown clinical significance) were detected by microarray analysis. Investigators concluded that microarray analysis may be especially useful in cases involving stillbirth (when a karyotype cannot be obtained) and structural abnormalities.
We want an accurate, completely risk-free genetic test that can be used for anyone. What we have so far is a technology that must be tested before it can be used in most of our patients—that is, the low-risk ones. We also have access to the fetus’ genetic code on a very specific level.
The total costs of such an approach—test, interpretation, counseling, and long-term follow-up of uncertain results—are unknown at this time and may prove to be unaffordable on a population-wide basis.
Should microarray analysis replace routine prenatal genetic testing?
A major dilemma associated with this technology is the significant amount of time that may be needed to counsel patients when the results are of unclear clinical significance.5 If the fetus has an anomaly, and a related CNV is identified, then counseling of the parents is fairly straightforward. However, if the fetus has an anomaly and a CNV that has not yet been defined, what should the parents be told? Some argue that this information should not be shared with the parents, whereas others recommend full disclosure of all results—even if we do not yet know what to make of them.
Another issue with microarray analysis is its inability to detect balanced translocations, triploidies, and low-level mosaicism, which require either a karyotype or whole-genome sequencing. Microarray analysis is also more expensive than karyotyping, although this may change in the future.
>Fetal therapy involves a complex equation of potential benefits and risks
Adzick NS, Thom EA, Spong CY, et al; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1104.
Fetal therapy is broadly defined as any intervention administered to or via the mother with a primary indication to improve perinatal or long-term outcomes for the fetus or newborn. The concept of intervening to prevent the death of a fetus by correcting an anatomic anomaly or halting a disease process in utero is not new. Liley performed the first intrauterine fetal transfusion for Rh alloimmunization in the 1960s. Today, we perform fetal interventions routinely to reduce mortality by giving medical therapy to the mother, such as antenatal corticosteroids to enhance fetal lung maturity or anti-arrhythmics for supraventricular tachycardia. More invasive procedures have proved to be lifesaving (placental laser coagulation for twin-twin transfusion syndrome), ameliorating in the short term (shunting for lower urinary tract obstruction to relieve oligohydramnios), or ultimately not helpful (decompression of hydrocephalus).
Most recently, open fetal surgery has taken center stage as an intervention focused not only on reducing mortality but on improving function and quality of life for fetuses with open neural tube defects (ONTDs). This anomaly was targeted for fetal intervention because, although ONTDs are not generally considered lethal, a significant number of patients die before the age of 5, the majority of patients require shunts that leave them vulnerable to complications, and ONTDs generally impose lifelong intellectual and physical limitations. Repair during fetal life was proposed to prevent damage to the spinal cord and reverse hindbrain herniation, with the goal of improving long-term neurologic function.
The Management of Myelomeningocele Study (MOMS) is a prospective, multicenter trial that randomly assigned fetuses with isolated ONTDs to open fetal repair of myelomeningocele via hysterotomy or to postnatal repair of the defect. Forty percent of infants who underwent fetal repair required placement of a shunt, compared with 82% of those who had postnatal repair (relative risk, 0.48; 97.7% CI, 0.36 to 0.64; P<.001). Infants in the fetal-repair group also had significantly improved composite scores for mental development and motor function at 30 months (P = .007), as well as improvement in secondary outcomes such as hindbrain herniation and independent walking at 30 months.
As exciting as these results are, open fetal surgery still has significant limitations. The few centers that perform the most complex surgeries often have strict exclusion criteria, including maternal body mass index (BMI) greater than 35 kg/m2 and other medical comorbidities. The surgery also poses real risks for both mother and fetus. In the MOMS trial, the risk of preterm labor increased in the fetal-repair group, compared with postnatal repair (38% vs 14%), as did the risk of premature rupture of membranes (46% vs 8%). The fetal-repair group delivered more than 3 weeks earlier than the postnatal repair group (34 vs 37 weeks). Twenty-five percent of the fetal-repair group had thinning of the uterine scar, with uterine dehiscence seen in 10%. When myelomeningocele is repaired during fetal life, mothers require two hysterotomies during pregnancy and face an increased risk of uterine rupture and preterm delivery in subsequent pregnancies. The use of tocolytics exposes these mothers to an increased risk of pulmonary edema (6% in the fetal-repair group vs 0% for postnatal repair).
Other issues that should be addressed:
- the need for rigorous study of open fetal surgery for other fetal anomalies
- prognostic factors for success and for complications
- long-term outcomes in neurologic development of children and fertility of mothers
- a comparison of costs between fetal and postnatal treatment.
Although we may want to intervene as early in life as possible (that is, the fetal period) to achieve the best outcomes for the child, we need to weigh the short-term benefits of intervention against the known risks that intervention poses for the mother in the current pregnancy as well as the potential implications for future pregnancies (ie, the need for all future deliveries to be by cesarean section), not to mention the unknown long-term effects of intervention on both the child and society.
Bundled payments may help us deliver higher-quality, more efficient, and less costly care
Centers for Medicare and Medicaid Services, US Department of Health and Human Services. Fact Sheet: Bundled Payments for Care Improvement Initiative. Washington, DC: DHS; 2011. http://innovations.cms.gov/Files/fact-sheet/Bundled-Payment-Fact-Sheet.pdf. Accessed December 6, 2012.
There is little question that health care in the United States needs reform. The culture of “more is better” is not sustainable economically—nor does our health as a whole reflect the amount of money that we spend on health care, compared with other countries. Although the future is not yet clear, one proposed mechanism for reform is the institution of bundled payments—the grouping of multiple services into one “episode” for payment purposes. An episode might include inpatient hospitalization for pneumonia, for example, or the grouping of surgery with post-discharge care. In obstetrics, all pregnancy care could be grouped into one episode. The concept behind bundled payments is to provide incentives to institutions and providers to delivery higher-quality, more efficient, and less costly care.
If bundled payments become the reality for obstetric care in the future, how will that affect the way we care for our patients? Instead of blindly ordering all available tests, we need to consider thoroughly whether the patient truly needs a test to improve pregnancy outcomes. We also need to consider whether other measures might be avoided safely to keep costs within the bundle. A few examples:
- Is a screening fetal echocardiogram really necessary in a diabetic woman if the ultrasound anatomy scan is sufficient to rule out any cardiac anomaly that might require intervention in the delivery room?
- How will we integrate the expense of cell-free fetal DNA aneuploidy testing and microarray analysis, not to mention the extended counseling sessions that will be necessary to explain findings of uncertain clinical significance, into the bundle? Will “low-risk” patients need to pay out of pocket?
Few physicians entered medicine to worry about costs. Most of us want to worry about our patients. Yet, the reality is that scientific curiosity and a desire to do more—and to do it sooner, faster, and safer—are no longer sufficient justifications for many clinical decisions. We soon may need to figure out how to get what we need without spending as much in the process. In doing so, we may find ourselves moving away from the computer screen and back to the bedside—where we belong.
- Will a second ultrasound scan to visualize the fetal spine in a patient with a normal alpha-fetoprotein level be included in the bundle or paid for by the patient?
These issues may seem trivial, but we can no longer afford to order every test available. We will need to spend more time examining and counseling our patients so that they feel they are still getting the best care possible.
We want to hear from you! Tell us what you think.
1. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis by fetal nucleic acid analysis in maternal plasma: the coming of age. Semin Fetal Neonatal Med. 2011;16(2):88-93.
2. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis empowered by high-throughput sequencing. Prenat Diagn. 2012;32(4):401-406.
3. Savage MS, Mourad MJ, Wapner RJ. Evolving applications of microarray analysis in prenatal diagnosis. Curr Opin Obstet Gynecol. 2011;23(2):103-108.
4. Dugoff L. Application of genomic technology in prenatal diagnosis [editorial]. N Engl J Med. 2012;367(23):2249-2251.
5. Wapner RJ, Driscoll DA, Simpson JL. Integration of microarray technology into prenatal diagnosis: counseling issues generated during the NICHD clinical trial. Prenat Diagn. 2012;32(4):396-400.
1. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis by fetal nucleic acid analysis in maternal plasma: the coming of age. Semin Fetal Neonatal Med. 2011;16(2):88-93.
2. Chiu RWK, Lo YMD. Noninvasive prenatal diagnosis empowered by high-throughput sequencing. Prenat Diagn. 2012;32(4):401-406.
3. Savage MS, Mourad MJ, Wapner RJ. Evolving applications of microarray analysis in prenatal diagnosis. Curr Opin Obstet Gynecol. 2011;23(2):103-108.
4. Dugoff L. Application of genomic technology in prenatal diagnosis [editorial]. N Engl J Med. 2012;367(23):2249-2251.
5. Wapner RJ, Driscoll DA, Simpson JL. Integration of microarray technology into prenatal diagnosis: counseling issues generated during the NICHD clinical trial. Prenat Diagn. 2012;32(4):396-400.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.