User login
Cardiovascular Disease and Risk of Hip Fracture
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673.
OTC Analgesics Not Associated with Acute Decompensation in Cirrhotic Patients
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Lower Perioperative Mortality with Endovascular Vs. Open Abdominal Aortic Aneurysm Repair
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
Discontinuation of Beta Blockers Increases Risk of Postoperative Myocardial Infarction and Death
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Cancer Guideline for VTE Prophylaxis for Inpatients and Long-Term Treatment With Low-Molecular-Weight Heparin for Acute VTE
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Trauma Patients with Pulmonary Embolism Might Not Have DVT on Imaging of Lower Extremities
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Eliminating Adverse Events and Redundant Tests Could Generate U.S. Healthcare Savings
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
High Perioperative Oxygen Fraction Does Not Improve Surgical-Site Infection Frequency after Abdominal Surgery
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
How Should Hyponatremia Be Evaluated and Managed?
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
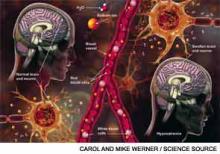
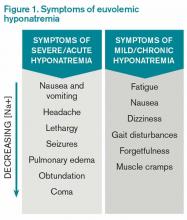
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
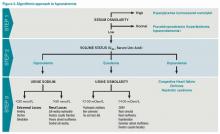
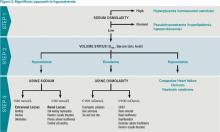
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
In the Literature: February 2010
In This Edition
Literature at a Glance
A guide to this month’s studies
- Perioperative oxygen use and infection rate.
- Effects of adverse events and healthcare costs
- Impact of DVT on PE rates in trauma
- VTE prevention and treatment in cancer patients
- Effect of perioperative beta-blocker discontinuation
- Endovascular vs. open AAA repair
- OTC analgesics in patients with hepatic dysfunction
- Cardiovascular disease and risk of hip fracture
High Perioperative Oxygen Fraction Does Not Improve Surgical-Site Infection Frequency after Abdominal Surgery
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
Eliminating Adverse Events and Redundant Tests Could Generate U.S. Healthcare Savings
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
Trauma Patients with Pulmonary Embolism Might Not Have DVT on Imaging of Lower Extremities
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Cancer Guideline for VTE Prophylaxis for Inpatients and Long-Term Treatment With Low-Molecular-Weight Heparin for Acute VTE
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Discontinuation of Beta Blockers Increases Risk of Postoperative Myocardial Infarction and Death
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Lower Perioperative Mortality with Endovascular Vs. Open Abdominal Aortic Aneurysm Repair
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
OTC Analgesics Not Associated with Acute Decompensation in Cirrhotic Patients
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Cardiovascular Disease and Risk of Hip Fracture
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Perioperative oxygen use and infection rate.
- Effects of adverse events and healthcare costs
- Impact of DVT on PE rates in trauma
- VTE prevention and treatment in cancer patients
- Effect of perioperative beta-blocker discontinuation
- Endovascular vs. open AAA repair
- OTC analgesics in patients with hepatic dysfunction
- Cardiovascular disease and risk of hip fracture
High Perioperative Oxygen Fraction Does Not Improve Surgical-Site Infection Frequency after Abdominal Surgery
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
Eliminating Adverse Events and Redundant Tests Could Generate U.S. Healthcare Savings
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
Trauma Patients with Pulmonary Embolism Might Not Have DVT on Imaging of Lower Extremities
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Cancer Guideline for VTE Prophylaxis for Inpatients and Long-Term Treatment With Low-Molecular-Weight Heparin for Acute VTE
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Discontinuation of Beta Blockers Increases Risk of Postoperative Myocardial Infarction and Death
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Lower Perioperative Mortality with Endovascular Vs. Open Abdominal Aortic Aneurysm Repair
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
OTC Analgesics Not Associated with Acute Decompensation in Cirrhotic Patients
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Cardiovascular Disease and Risk of Hip Fracture
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Perioperative oxygen use and infection rate.
- Effects of adverse events and healthcare costs
- Impact of DVT on PE rates in trauma
- VTE prevention and treatment in cancer patients
- Effect of perioperative beta-blocker discontinuation
- Endovascular vs. open AAA repair
- OTC analgesics in patients with hepatic dysfunction
- Cardiovascular disease and risk of hip fracture
High Perioperative Oxygen Fraction Does Not Improve Surgical-Site Infection Frequency after Abdominal Surgery
Clinical question: Does the use of 80% oxygen perioperatively in abdominal surgery decrease the frequency of surgical-site infection within 14 days without increasing the rate of pulmonary complications?
Background: Low oxygen tension in wounds can negatively impact immune response and healing. Increasing inspiratory oxygen fraction during the perioperative period translates into higher wound oxygen tension. However, the benefit of increased oxygen fraction therapy in abdominal surgery healing and complications is not clear, nor is the frequency of pulmonary complications.
Study design: Patient- and observer-blinded clinical trial.
Setting: Fourteen Danish hospitals from October 2006 to October 2008.
Synopsis: Patients were randomized to receive a fraction of inspired oxygen (FIO2) of 0.80 or 0.30. The primary outcome—surgical-site infection in the superficial or deep wound or intra-abdominal cavity within 14 days of surgery—was defined using Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included pulmonary complications within 14 days (pneumonia, atelectasis, or respiratory failure), 30-day mortality, duration of post-op course, ICU stay within 14 days post-op, and any abdominal operation within 14 days. The 1,386 patients were enrolled in the intention-to-treat analysis.
Infection occurred in 19.1% of patients given 0.80 FIO2 and in 20.1% of patients given 0.30 FIO2; odds ratio of 0.94 (95% CI 0.72 to 1.22; P=0.64). Numbers of pulmonary complications were not significantly different between the groups.
This trial included acute and nonacute laparotomies with followup for adverse outcomes. Study limitations included the inability to ensure that both groups received timely antibiotics and prevention for hypothermia. Of patients in the 30% FIO2 group, 7.3% required higher oxygen administration. Additionally, infection might have been underestimated in 11.3% of patients who were not followed up on between days 13 and 30.
Bottom line: High oxygen concentration administered during and after laparotomy did not lead to fewer surgical site infections, nor did it significantly increase the frequency of pulmonary complications or death.
Citation: Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302(14):1543-1550.
Eliminating Adverse Events and Redundant Tests Could Generate U.S. Healthcare Savings
Clinical question: Using available data, what is the estimated cost savings of eliminating adverse events and avoiding redundant tests?
Background: Reimbursement schemes are changing such that hospitals are reimbursed less for some adverse events. This financial disincentive is expected to spark interest in improved patient safety. The authors sought to model the cost savings generated by eliminating redundant testing and adverse events from literature-based estimates.
Study design: Development of conceptual model to identify common or costly adverse events, redundant tests, and simulated costs.
Setting: Literature review, expert opinion, data from safety organizations and epidemiologic studies, and patient data from the 2004 National Inpatient Data Sample.
Synopsis: The conceptual model identified 5.7 million adverse events in U.S. hospitals, of which 3 million were considered preventable. The most common events included hospital-acquired infections (82% preventable), adverse drug events (26%), falls (33%), and iatrogenic thromboembolic events (62%). The calculated cost savings totaled $16.6 billion (5.5% of total inpatient costs) for adverse events and $8.2 billion for the elimination of redundant tests. When looking at hospital subtypes, the greatest savings would come from major teaching hospitals.
This study is limited by its use of published and heterogeneous data spanning a 15-year period. The authors did not include events for which there was no epidemiologic or cost data. As hospital-care changes and technology is adopted, it is uncertain how this changes the costs, prevalence, and the preventable nature of these events. The model was not consistently able to identifying high- and low-risk patients. For instance, in some models, all patients were considered at risk for events.
Bottom line: Based on a conceptual model of 2004 hospitalized patients, eliminating preventable adverse events could have saved $16.6 billion, while eliminating redundant tests could have saved another $8 billion.
Citation: Jha AK, Chan DC, Ridgway AB, Franz C, Bates DW. Improving safety and eliminating redundant tests: cutting costs in U.S. hospitals. Health Aff (Millwood). 2009;28(5):1475-1484.
Trauma Patients with Pulmonary Embolism Might Not Have DVT on Imaging of Lower Extremities
Clinical question: What is the relationship between acute DVT and pulmonary embolism (PE) in trauma patients?
Background: Major trauma is associated with an increased risk of acute DVT and PE. It is assumed that the majority of PEs arise from DVTs in the lower extremities. Definitive evidence demonstrating that PEs form in situ rather than embolize from leg veins could impact indications for inferior vena cava filters.
Study design: Retrospective chart review.
Setting: Academic Level 1 trauma center in Boston.
Synopsis: The medical records of 247 trauma patients with suspected PE who underwent CT angiography of the lungs and simultaneous CT venography of the pelvis and lower extremities from January 2004 to December 2007 were reviewed. High-risk patients also underwent weekly screening with duplex ultrasonagraphy of the legs.
PE was diagnosed in 46 patients (19%) and DVT in 18 patients (7%). Anticoagulant prophylaxis had been administered to 96% and 78% of the patients with PE and DVT, respectively. PE was diagnosed a median of 5.5 days after admission (range 0-40 days) and the majority (61%) were in segmental or subsegmental branches, rather than in the main or lobar pulmonary arteries (39%). Only seven of the 46 patients (15%) diagnosed with PE also had a pelvic or lower-extremity DVT on simultaneous imaging with CT venography.
Bottom line: Trauma patients with PE often do not have a DVT at the time of diagnosis, though it remains unknown whether this is due to in-situ pulmonary thrombosis or complete embolization from the lower extremities.
Citation: Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928-932.
Cancer Guideline for VTE Prophylaxis for Inpatients and Long-Term Treatment With Low-Molecular-Weight Heparin for Acute VTE
Clinical question: On what aspects of VTE management in cancer patients are there consensus among the major guideline panels?
Background: VTE is a common and serious complication of cancer. Patients might be hypercoagulable due to prothrombotic mediators released or mediated by tumor cells, chemotherapeutic agents, debility, central venous catheters, hospitalizations, or surgical procedures. The optimal management often is problematic due to uncertain benefit and risk of bleeding.
Study design: Review of major guideline statements.
Synopsis: The authors examined five VTE guidelines of American and European cancer societies. Each guideline was reviewed to determine the main recommendations and whether there was consensus on key aspects of anticoagulant management.
The study authors concluded that consensus was reached on most key recommendations:
- VTE prophylaxis in hospitalized medical patients. All five guidelines recommend the use of prophylaxis, though some guidelines recommend anticoagulant prophylaxis for all inpatients in the absence of contraindications and some recommend limiting prophylaxis to immobilized patients. All five recommend the use of either unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.
- VTE prevention in cancer patients undergoing surgery. All five guidelines recommend anticoagulant prophylaxis in the absence of contraindications and extending prophylaxis approximately four weeks after major surgery.
- VTE prophylaxis in cancer patients with central venous catheters. Not recommended.
- VTE prophylaxis in ambulatory cancer patients without central venous catheters. Recommended only for multiple myeloma patients receiving a thalidomide-lenalidomide regimen.
- Long-term treatment of acute VTE in cancer patients. All five guidelines recommend initial treatment with LMWH for at least three to six months, followed by indefinite treatment with LMWH or a vitamin K antagonist.
Bottom line: Major guideline panels agree on key aspects of VTE management for cancer patients, including the use of prophylaxis for hospitalized medical and surgical patients and the use of long-term LMWH treatment for cancer patients with acute VTE.
Citation: Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009; 27(29):4919-4926.
Discontinuation of Beta Blockers Increases Risk of Postoperative Myocardial Infarction and Death
Clinical question: Does perioperative beta-blocker discontinuation affect postoperative myocardial infarction (MI) in low-risk patients undergoing joint arthroplasty?
Background: Recent trials show no benefit of perioperative beta blockers in reducing the incidence of perioperative myocardial infarctions (POMI) in low-risk patients. This retrospective study examined the impact of continuing or discontinuing beta blockers and the occurrence of POMI in patients undergoing elective joint arthroplasties.
Study design: Retrospective chart review.
Setting: Large academic center in Ottawa, Canada.
Synopsis: Medical records for 5,178 patients undergoing elective hip or knee arthroplasty from January 2002 to June 2006 were included in the review. The primary outcome was POMI, defined as an increased troponin level. Patients were divided into three groups: beta blocker prescribed on post-operative day (POD) zero and continued for one week or until discharge; beta blocker prescribed on POD zero and discontinued at any time in the first week; and no beta blocker on POD 0.
Beta blockers were continued in 992 patients and discontinued in 252 patients. The rate of POMI and death increased in the beta-blocker discontinuation group (odds ratio 2.0 [1.1-3.9] and 2.0 [1.1-3.9], respectively). This association persisted after adjustment for cardiac risk using a validated risk score.
The study was limited by the fact that the control group did not include patients who were on a beta blocker at home, thus potentially increasing the number of events in this group. The discontinuation beta blocker group had an increased baseline risk for POMI. The reason for discontinuing the beta blocker was not known, and cessation of beta blocker could have been due to an acute event.
Bottom line: This study adds support to the American College of Cardiology and American Heart Association (ACC/AHA) guidelines, which recommend continuation of beta-blocker therapy in the perioperative period.
Citation: Van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology. 2009;111(4):717-724.
Lower Perioperative Mortality with Endovascular Vs. Open Abdominal Aortic Aneurysm Repair
Clinical question: How do perioperative and long-term morbidity and mortality compare in endovascular and open repair of abdominal aortic aneurysm (AAA)?
Background: Open AAA repair has relatively high perioperative mortality. Endovascular repair was developed as a less-invasive option and has been shown to reduce inpatient perioperative mortality, length of hospital stay, and ICU requirement. However, data suggest it leads to more frequent reinterventions and the same mortality rate as open repair at two years.
Study design: Randomized clinical trial.
Setting: Veterans Affairs medical centers.
Synopsis: The study randomized 881 veterans who planned to have elective AAA repair and were eligible for both endovascular and open repair. This is a planned, two-year interim report in a nine-year study.
Perioperative mortality was 0.5% in the endovascular repair group, compared with 3.0% in the open repair group. However, this difference in mortality was not statistically significant at two years. The endovascular repair group experienced shorter procedure and mechanical ventilation time, decreased hospital and ICU stay, and lower rate of blood transfusions.
Overall, there was no difference between the groups for major morbidity, procedure failure, need for secondary therapeutic intervention, quality of life, or erectile dysfunction. More data on long-term comparison of these two interventions will be available at the conclusion of this study.
Bottom line: Endovascular repair of AAA has lower perioperative mortality than open repair but did not lead to improved morbidity or mortality at two years.
Citation: Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs. open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302 (14):1535-1542.
OTC Analgesics Not Associated with Acute Decompensation in Cirrhotic Patients
Clinical question: Do over-the-counter (OTC) analgesics lead to acute hepatic decompensation among patients with cirrhosis?
Background: In theory, intake of acetaminophen and/or nonsteroidal anti-inflammatory drugs (NSAIDs) can worsen hepatic function and lead to complications among cirrhotic patients. The role of OTC analgesics in potentially triggering acute hepatic decompensation among cirrhotic patients has not been studied.
Study design: Prospective case-control study.
Setting: Two tertiary-care hospitals.
Synopsis: Cirrhotic patients hospitalized for acute liver decompensation were compared with compensated cirrhotic patients in the liver clinic (cirrhotic controls) and with randomly selected, noncirrhotic patients who were simultaneously hospitalized (noncirrhotic controls). Data collected through questionnaires included quantity and dose of OTC analgesics used and alcohol consumption in the past 30 days.
Thirty-five percent of the hospitalized cirrhotic patients, 52% of the cirrhotic controls, and 70% of the noncirrhotic controls used OTC analgesics. At doses lower than those recommended, acetaminophen is not associated with acute liver decompensation among cirrhotic patients, even with recent alcohol use. However, NSAIDs taken by the cirrhotic patients, when compared to control subjects, were in larger doses and used for a longer duration, suggesting NSAIDs may have contributed to the acute decompensation.
Study limitations include the nature of the study design, reliance on the patient’s recall of OTC analgesic use, and obtaining other possible causes of decompensation, such as herbal supplement intake or compliance with diuretics or dietary indiscretion.
Bottom line: Acetaminophen at doses lower than recommended is not associated with adverse complications in cirrhotic patients, but NSAIDs are possibly associated with acute decompensation.
Citation: Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7(9):994-999.
Cardiovascular Disease and Risk of Hip Fracture
Clinical question: Is the diagnosis of cardiovascular disease (CVD) associated with the risk of subsequent hip fracture?
Background: Osteoporosis and CVD are regarded as independent, age-related conditions. However, recent research suggests that the bone and vascular systems share common regulatory mechanisms. Stroke is a known risk factor for hip fractures, and bisphosphonates have been shown to prevent atherosclerosis and reduce total mortality rate.
Study design: Cohort study.
Setting: Swedish National Patient Registry.
Synopsis: The study identified 31,936 Swedish twins born from 1914 to 1944. This cohort was followed up to age 50, and time-dependent exposures using Cox-proportional hazard regression models were evaluated.
Times to hip fracture after CVD diagnosis were isolated. Crude absolute rate of hip fractures (per 1,000 person-years) was 12.6 after diagnosis of heart failure, 12.6 after a stroke, 6.6 after peripheral atherosclerosis, and 5.2 after ischemic heart disease (IHD), compared with 1.2 per 1,000 person-years without a CVD diagnosis. Multivariable-adjusted hazard ratio (HR) of hip fracture after heart failure was 4.40 (95% CI, 3.43-5.63); after a stroke was 5.09 (95% CI, 4.18-6.20); after peripheral atherosclerosis was 3.20 (CI, 2.28-4.50); and after an IHD event was 2.32 (CI, 1.91-2.84).
Identical twins even without heart failure and stroke also had an increased risk of hip fracture if their twin had been diagnosed with these diseases.
Bottom line: Cardiovascular disease is significantly associated with risk of subsequent hip fracture, and genetic factors probably play a role in the association.
Citation: Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666-1673. TH